Bionomics IPO Presentation Deck
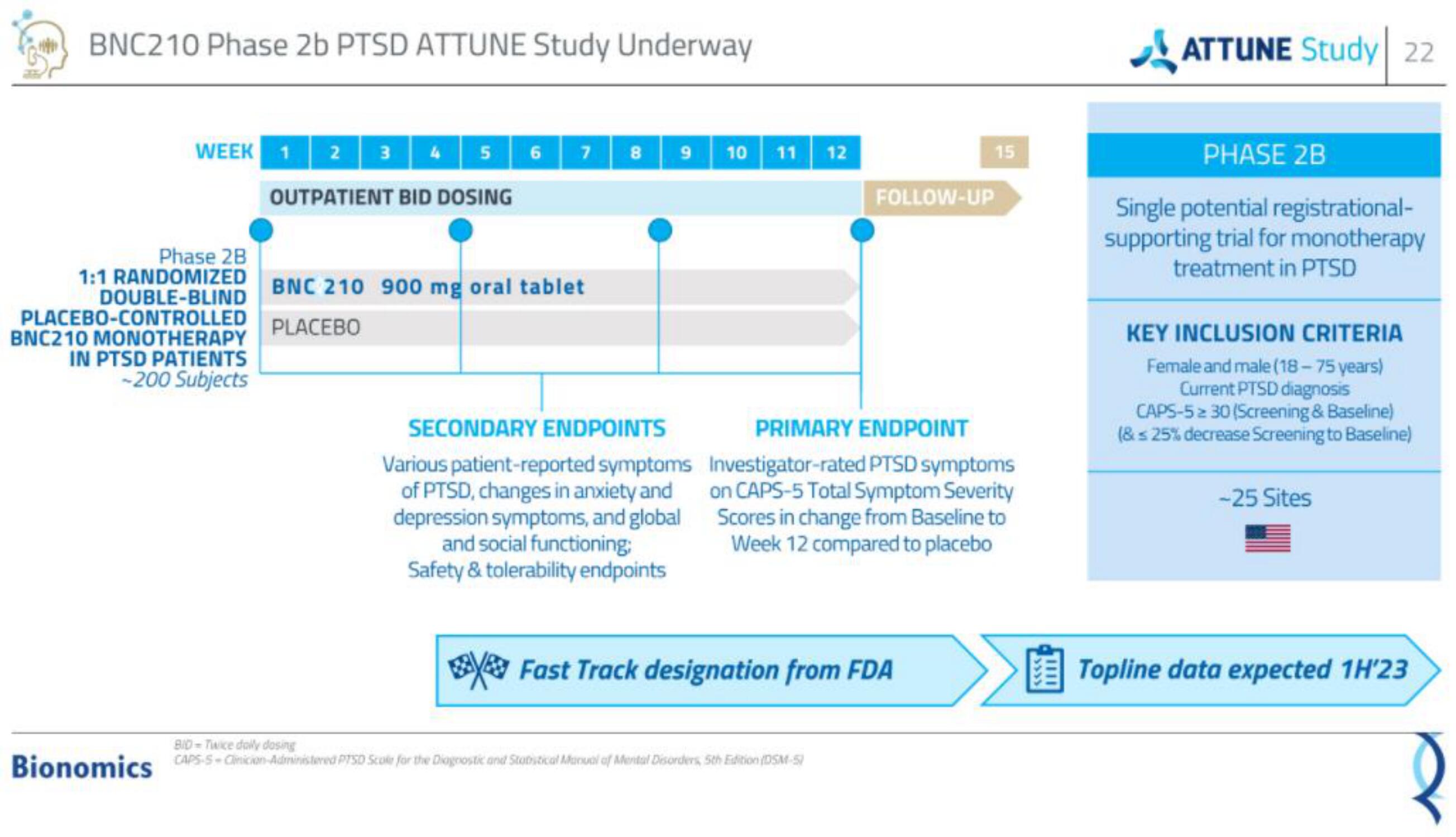
BNC210 Phase 2b PTSD ATTUNE Study Underway
WEEK1 2 3 4 5 6 7
OUTPATIENT BID DOSING
Phase 2B
1:1 RANDOMIZED
DOUBLE-BLIND
PLACEBO-CONTROLLED
BNC210 MONOTHERAPY
IN PTSD PATIENTS
-200 Subjects
BNC 210 900 mg oral tablet
PLACEBO
8
9
SECONDARY ENDPOINTS
Various patient-reported symptoms
of PTSD, changes in anxiety and
depression symptoms, and global
and social functioning:
Safety & tolerability endpoints
10 11 12
FOLLOW-UP
PRIMARY ENDPOINT
Investigator-rated PTSD symptoms
on CAPS-5 Total Symptom Severity
Scores in change from Baseline to
Week 12 compared to placebo
Fast Track designation from FDA
BID-Twice dolly dosing
Bionomics CAPS-S-Clinicion Administered P750 Scole for the Diagnostic and Stonitical Manual of Mental Disorders, Sch Edition (DSM-S)
15
ATTUNE Study 22
PHASE 2B
Single potential registrational-
supporting trial for monotherapy
treatment in PTSD
KEY INCLUSION CRITERIA
Female and male (18-75 years)
Current PTSD diagnosis
CAPS-5 ≥ 30 (Screening & Baseline)
(& ≤ 25% decrease Screening to Baseline)
-25 Sites
欧 Topline data expected 1H'23View entire presentation