Kymera Investor Day Presentation Deck
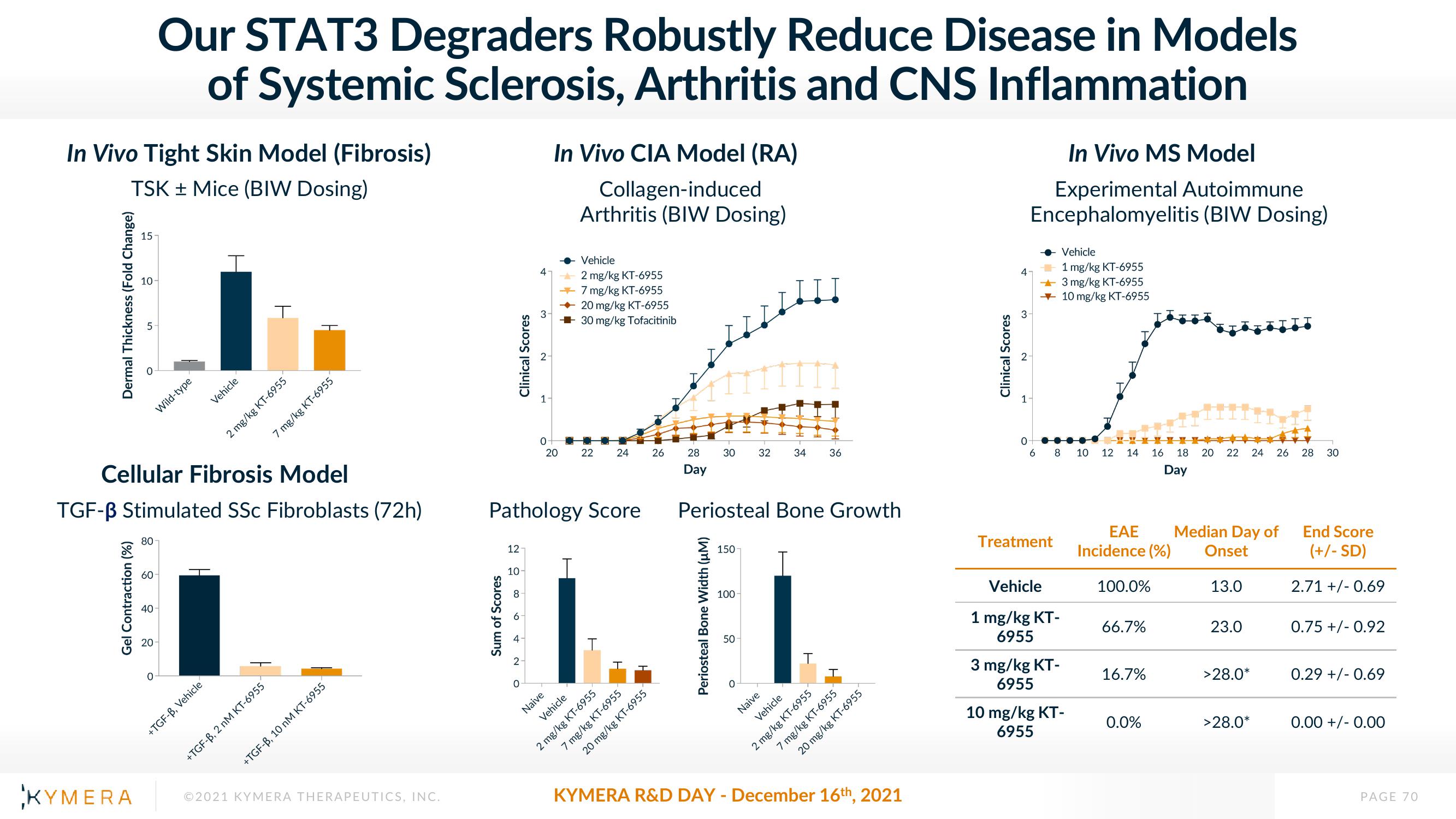
In Vivo Tight Skin Model (Fibrosis)
TSK + Mice (BIW Dosing)
Dermal Thickness (Fold Change)
15
Gel Contraction (%)
10-
80
60
Our STAT3 Degraders Robustly Reduce Disease in Models
of Systemic Sclerosis, Arthritis and CNS Inflammation
40
20
Wild-type
Cellular Fibrosis Model
TGF-B Stimulated SSC Fibroblasts (72h)
O
Vehicle
+TGF-B, Vehicle
T
+TGF-B, 2 nM KT-6955
2 mg/kg KT-6955
7 mg/kg KT-6955
+TGF-B, 10 nM KT-6955
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
Sum of Scores
Clinical Scores
12-
10
∞06+
4
O
3
N
Pathology Score
1
In Vivo CIA Model (RA)
Collagen-induced
Arthritis (BIW Dosing)
Vehicle
2 mg/kg KT-6955
→7 mg/kg KT-6955
→
20 mg/kg KT-6955
30 mg/kg Tofacitinib
0
20 22 24 26
Naive
Vehicle
2 mg/kg KT-6955
7 mg/kg KT-6955
20 mg/kg KT-6955
28
Day
30 32
Periosteal Bone Width (µM)
150
Periosteal Bone Growth
100
50
Naive
34
Vehicle
+
36
2 mg/kg KT-6955
7 mg/kg KT-6955
20 mg/kg KT-6955
KYMERA R&D DAY - December 16th, 2021
Clinical Scores
4
3
N
1
0
In Vivo MS Model
Experimental Autoimmune
Encephalomyelitis (BIW Dosing)
6
Treatment
8
Vehicle
1 mg/kg KT-
6955
Vehicle
1 mg/kg KT-6955
3 mg/kg KT-6955
10 mg/kg KT-6955
3 mg/kg KT-
6955
10 mg/kg KT-
6955
10 12 14 16
EAE
Incidence (%)
100.0%
66.7%
16.7%
18 20 22 24 26
Day
0.0%
Median Day of
Onset
13.0
23.0
>28.0*
>28.0*
28 30
End Score
(+/-SD)
2.71 +/-0.69
0.75 +/- 0.92
0.29 +/-0.69
0.00 +/- 0.00
PAGE 70View entire presentation