Valneva IPO Presentation Deck
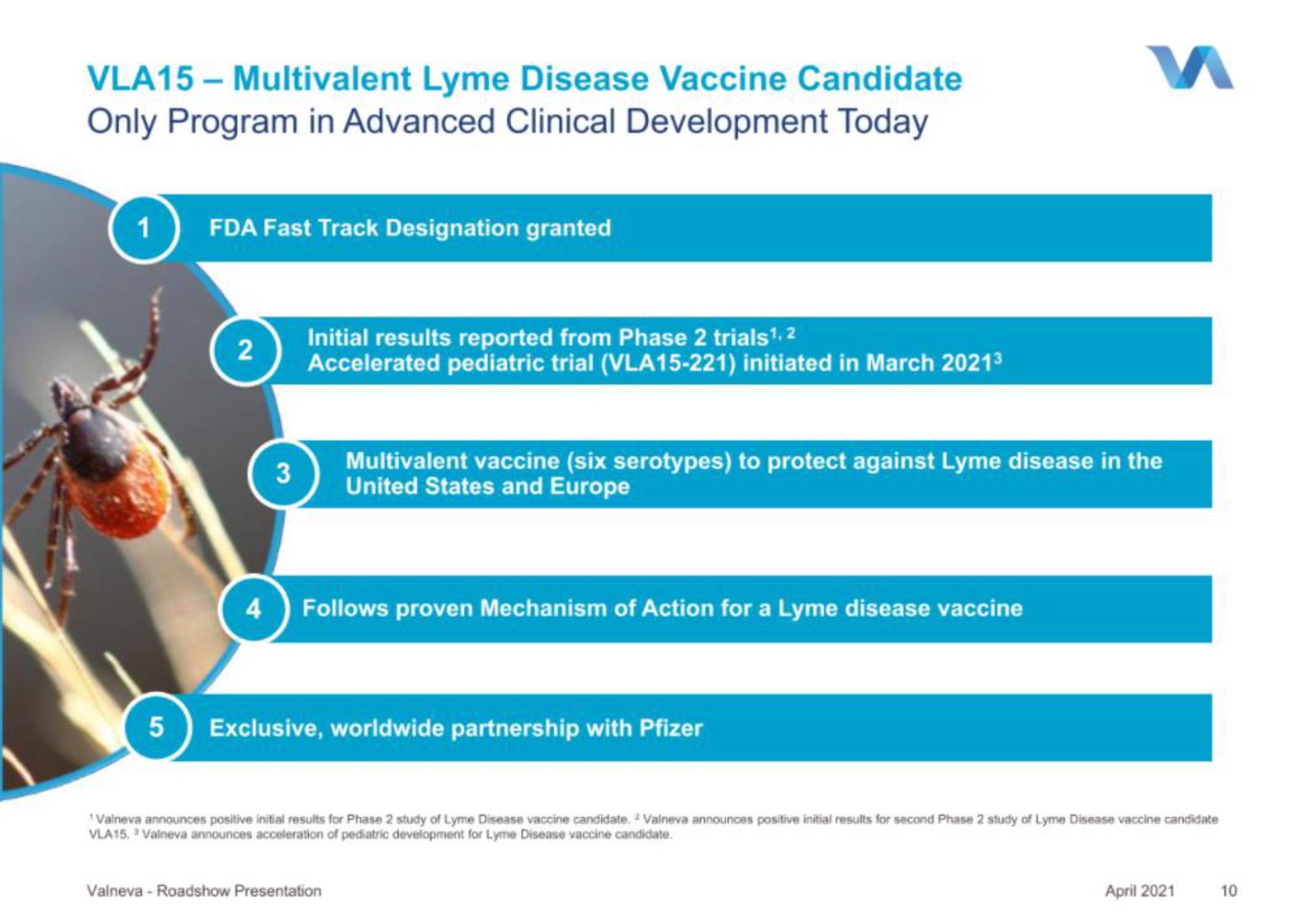
VLA15 - Multivalent Lyme Disease Vaccine Candidate
Only Program in Advanced Clinical Development Today
1 FDA Fast Track Designation granted
5
2
4
3
Initial results reported from Phase 2 trials ¹, 2
Accelerated pediatric trial (VLA15-221) initiated in March 2021³
Multivalent vaccine (six serotypes) to protect against Lyme disease in the
United States and Europe
Follows proven Mechanism of Action for a Lyme disease vaccine
Exclusive, worldwide partnership with Pfizer
Vaineva announces positive initial results for Phase 2 study of Lyme Disease vaccine candidate. 2 Vaineva announces positive initial results for second Phase 2 study of Lyme Disease vaccine candidate
VLA15,³ Valneva announces acceleration of pediatric development for Lyme Disease vaccine candidate.
Valneva - Roadshow Presentation
April 2021
10View entire presentation