LumiraDx Investor Presentation Deck
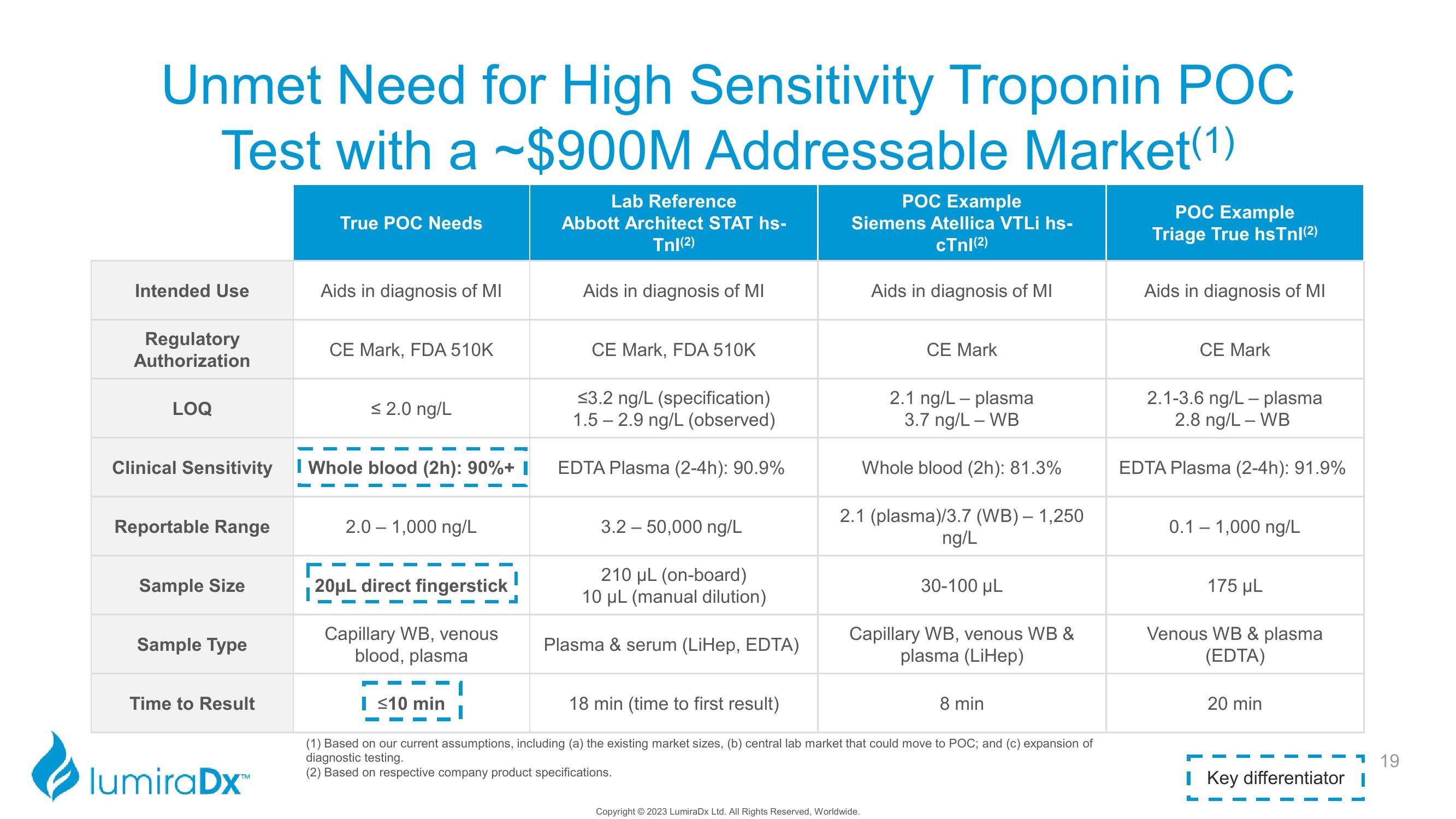
Unmet Need for High Sensitivity Troponin POC
Test with a ~$900M Addressable Market(1)
Intended Use
Regulatory
Authorization
LOQ
Clinical Sensitivity
Reportable Range
Sample Size
Sample Type
Time to Result
lumiraDx™
True POC Needs
Aids in diagnosis of MI
CE Mark, FDA 510K
≤ 2.0 ng/L
I Whole blood (2h): 90%+ I
2.0 − 1,000 ng/L
20μL direct fingerstick
Capillary WB, venous
blood, plasma
Lab Reference
Abbott Architect STAT hs-
Tnl(2)
I ≤10 min
Aids in diagnosis of MI
CE Mark, FDA 510K
≤3.2 ng/L (specification)
1.5-2.9 ng/L (observed)
EDTA Plasma (2-4h): 90.9%
3.2 – 50,000 ng/L
210 μL (on-board)
10 μL (manual dilution)
Plasma & serum (LiHep, EDTA)
POC Example
Siemens Atellica VTLi hs-
cTnl (2)
Aids in diagnosis of MI
CE Mark
2.1 ng/L plasma
3.7 ng/L - WB
Copyright © 2023 LumiraDx Ltd. All Rights Reserved, Worldwide.
Whole blood (2h): 81.3%
2.1 (plasma)/3.7 (WB) - 1,250
ng/L
30-100 μL
Capillary WB, venous WB &
plasma (LiHep)
18 min (time to first result)
(1) Based on our current assumptions, including (a) the existing market sizes, (b) central lab market that could move to POC; and (c) expansion of
diagnostic testing.
(2) Based on respective company product specifications.
8 min
POC Example
Triage True hsTnl (²)
Aids in diagnosis of MI
CE Mark
2.1-3.6 ng/L - plasma
2.8 ng/L - WB
EDTA Plasma (2-4h): 91.9%
0.1 – 1,000 ng/L
175 µL
Venous WB & plasma
(EDTA)
20 min
I Key differentiator I
19View entire presentation