Bausch+Lomb Results Presentation Deck
Launched Miebo in Q3, Strong Early Performance
NOW
AVAILABLE
NEW!
Miebo
(perfluorohexyloctane
ophthalmic solution)
BAUSCH+ LOMB
First-in-class prescription eye
drop approved for dry eye
disease (DED) that directly
targets tear evaporation
"Miebo is potentially a transformative
medication, a revolution in the dry eye space."
Darrell White, MD
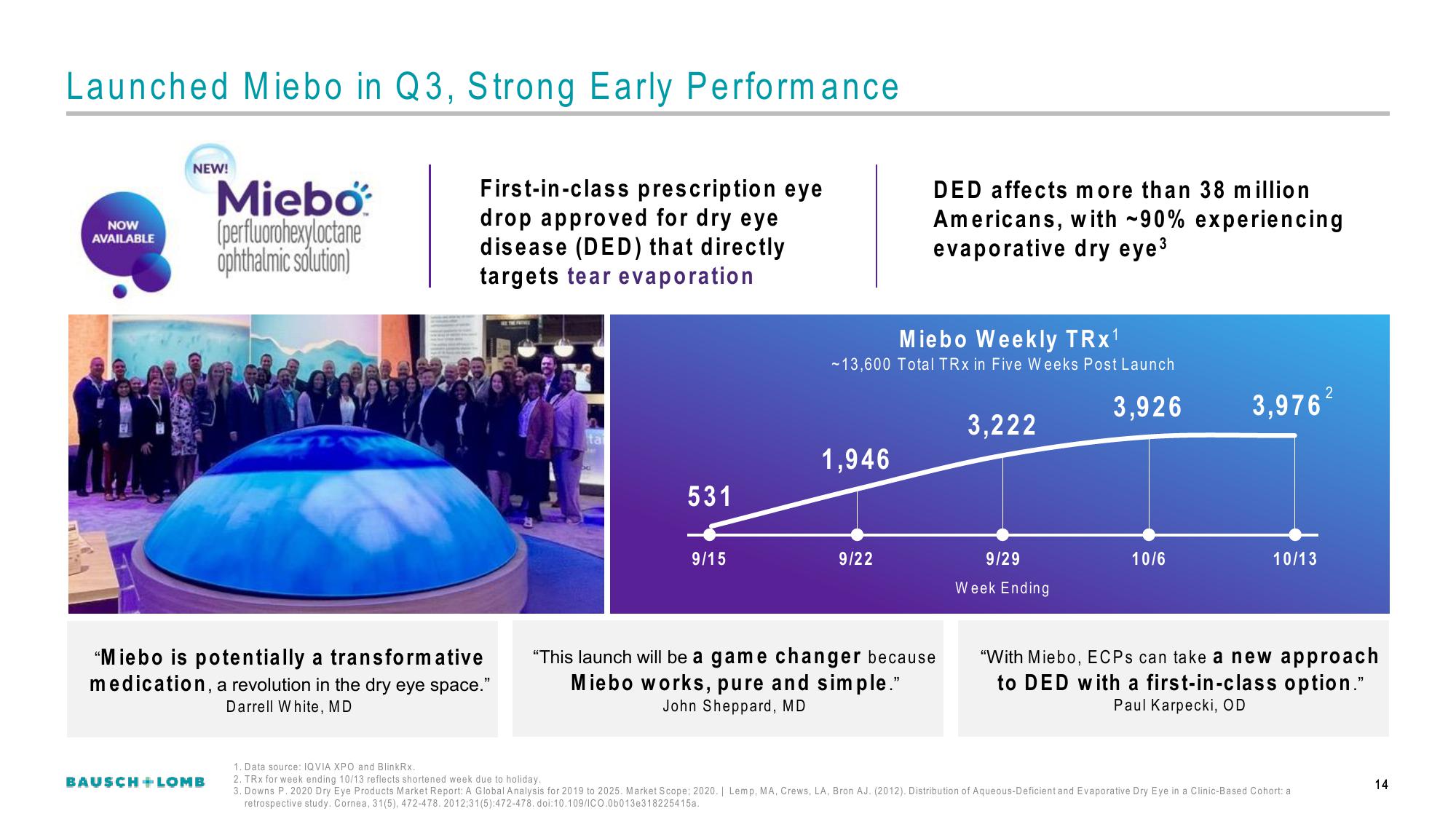
531
9/15
Miebo Weekly TRX¹
~13,600 Total TRX in Five Weeks Post Launch
3,926
1,946
DED affects more than 38 million
Americans, with ~90% experiencing
evaporative dry eye³
9/22
"This launch will be a game changer because
Miebo works, pure and simple."
John Sheppard, MD
3,222
9/29
Week Ending
10/6
3,976
10/13
2
"With Miebo, ECPs can take a new approach
to DED with a first-in-class option."
Paul Karpecki, OD
1. Data source: IQVIA XPO and BlinkRx..
2. TRX for week ending 10/13 reflects shortened week due to holiday.
3. Downs P. 2020 Dry Eye Products Market Report: A Global Analysis for 2019 to 2025. Market Scope; 2020. | Lemp, MA, Crews, LA, Bron AJ. (2012). Distribution of Aqueous-Deficient and Evaporative Dry Eye in a Clinic-Based Cohort: a
retrospective study. Cornea, 31(5), 472-478. 2012;31(5):472-478. doi:10.109/ICO.0b013e318225415a.
14View entire presentation