Immix Biopharma Investor Presentation Deck
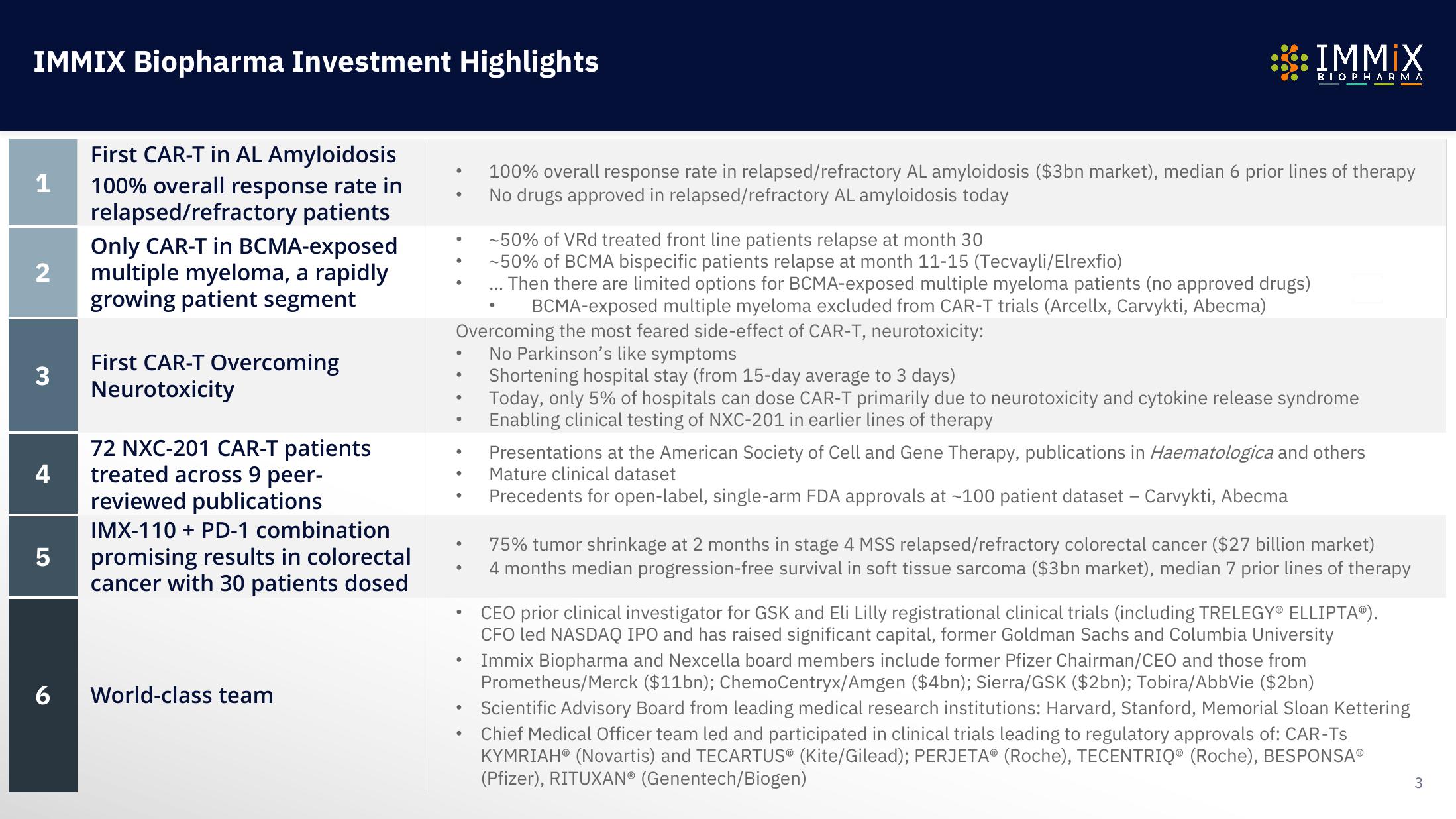
IMMIX Biopharma Investment Highlights
First CAR-T in AL Amyloidosis
1 100% overall response rate in
relapsed/refractory patients
Only CAR-T in BCMA-exposed
multiple myeloma, a rapidly
growing patient segment
2
3
72 NXC-201 CAR-T patients
4 treated across 9 peer-
reviewed publications
LO
5
First CAR-T Overcoming
Neurotoxicity
6
IMX-110 + PD-1 combination
promising results in colorectal
cancer with 30 patients dosed
World-class team
●
Overcoming the most feared side-effect of CAR-T, neurotoxicity:
No Parkinson's like symptoms
●
●
●
●
●
●●●
S
100% overall response rate in relapsed/refractory AL amyloidosis ($3bn market), median 6 prior lines of therapy
No drugs approved in relapsed/refractory AL amyloidosis today
-50% of VRd treated front line patients relapse at month 30
-50% of BCMA bispecific patients relapse at month 11-15 (Tecvayli/Elrexfio)
Then there are limited options for BCMA-exposed multiple myeloma patients (no approved drugs)
BCMA-exposed multiple myeloma excluded from CAR-T trials (Arcellx, Carvykti, Abecma)
IMMİX
BIOPHARMA
Shortening hospital stay (from 15-day average to 3 days)
Today, only 5% of hospitals can dose CAR-T primarily due to neurotoxicity and cytokine release syndrome
Enabling clinical testing of NXC-201 in earlier lines of therapy
Presentations at the American Society of Cell and Gene Therapy, publications in Haematologica and others
Mature clinical dataset
Precedents for open-label, single-arm FDA approvals at ~100 patient dataset - Carvykti, Abecma
75% tumor shrinkage at 2 months in stage 4 MSS relapsed/refractory colorectal cancer ($27 billion market)
4 months median progression-free survival in soft tissue sarcoma ($3bn market), median 7 prior lines of therapy
CEO prior clinical investigator for GSK and Eli Lilly registrational clinical trials (including TRELEGY® ELLIPTAⓇ).
CFO led NASDAQ IPO and has raised significant capital, former Goldman Sachs and Columbia University
Immix Biopharma and Nexcella board members include former Pfizer Chairman/CEO and those from
Prometheus/Merck ($11bn); ChemoCentryx/Amgen ($4bn); Sierra/GSK ($2bn); Tobira/AbbVie ($2bn)
Scientific Advisory Board from leading medical research institutions: Harvard, Stanford, Memorial Sloan Kettering
Chief Medical Officer team led and participated in clinical trials leading to regulatory approvals of: CAR-Ts
KYMRIAH® (Novartis) and TECARTUS® (Kite/Gilead); PERJETA® (Roche), TECENTRIQ® (Roche), BESPONSAⓇ
(Pfizer), RITUXAN® (Genentech/Biogen)
3View entire presentation