Neumora Therapeutics IPO Presentation Deck
Potential for Broad Applicability of Navacaprant Beyond MDD Includes
Bipolar Depression
Strong Rationale for Efficacy in Bipolar
Depression (BPD):
●
●
●
Evidence from the navacaprant Phase 2 study and the NIMH-
sponsored FASTMAS demonstrate the utility of KOR
pharmacology for depression and anhedonia symptoms
Anhedonia is a highly prevalent and clinically relevant symptom
in BPD, and there is a growing body of research in the
pathophysiologic underpinnings of anhedonia in BPD
Given that navacaprant studies have demonstrated meaningful
improvements in anhedonia symptoms in patients with
moderate-to-severe MDD, we believe it may also be effective in
treating anhedonia related to BPD
The primary endpoint for evaluating efficacy in bipolar
depression is MADRS
Currently approved therapies (e.g., atypical antipsychotics)
have significant limitations
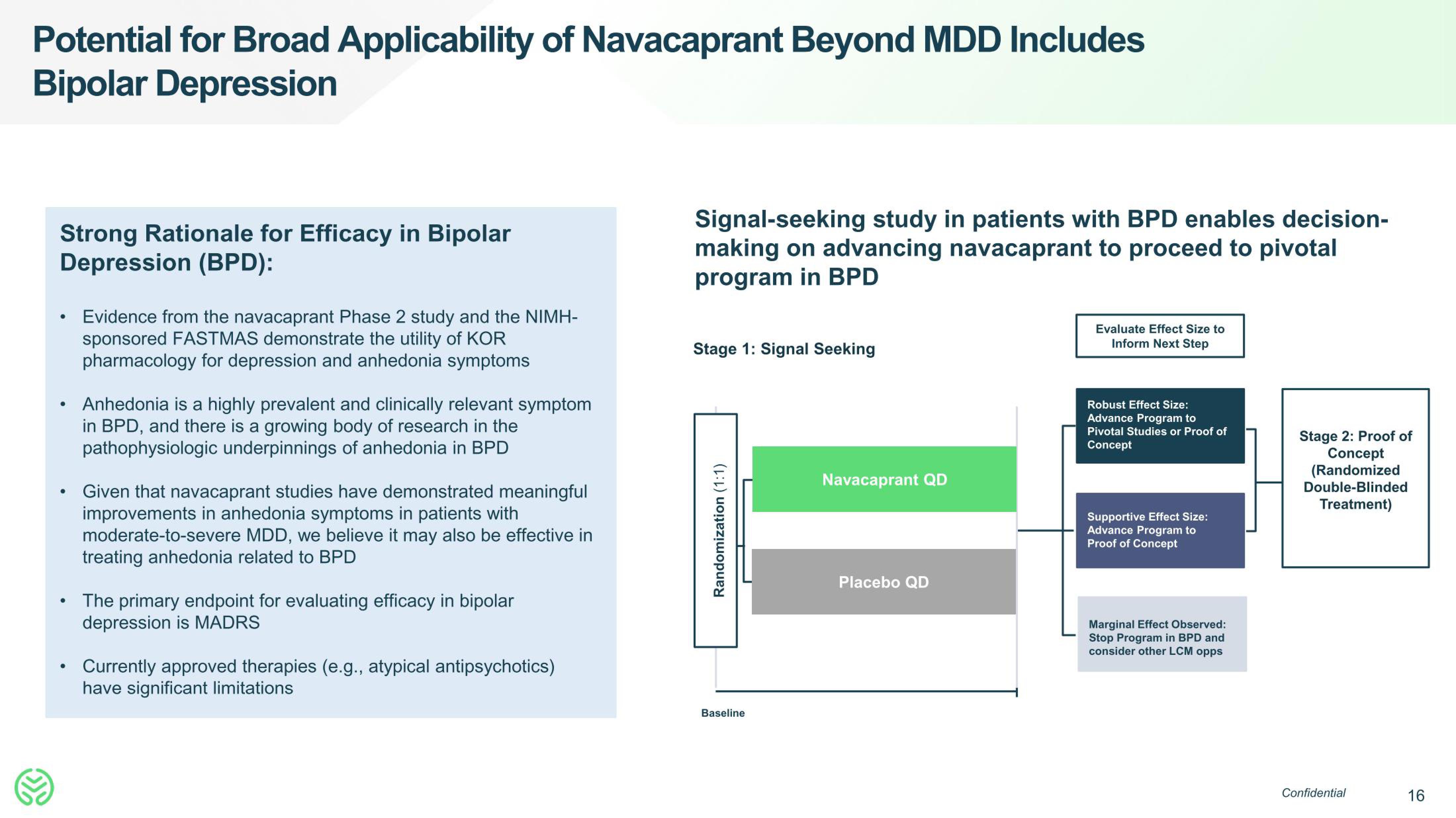
Signal-seeking study in patients with BPD enables decision-
making on advancing navacaprant to proceed to pivotal
program in BPD
Stage 1: Signal Seeking
Randomization (1:1)
Baseline
Navacaprant QD
Placebo QD
Evaluate Effect Size to
Inform Next Step
Robust Effect Size:
Advance Program to
Pivotal Studies or Proof of
Concept
Supportive Effect Size:
Advance Program to
Proof of Concept
Marginal Effect Observed:
Stop Program in BPD and
consider other LCM opps
Stage 2: Proof of
Concept
(Randomized
Double-Blinded
Treatment)
Confidential
16View entire presentation