Company Overview
For personal use only
(b) Frost & Sullivan Market Report as commissioned by APIRX, Sept. 2021,
market opportunity is medications
and other, where other includes visits to physicians, in/out patient costs
(c) Frost & Sullivan Market Report as commissioned by APIRX, Sept. 2021,
market opportunity is Adolescent
Substance Abuse
(g) ResearchandMarkets, "Outlook on the Glaucoma Therapeutics Global
Market", 2020-2026", Oct. 22. 2021
(o) Worldwide Market Reports, "Smoking Cessation and Nicotine De-Addiction
Products Market", May 2018
(p) Future Market Insights, "Dry Eye Syndrome Treatment Market", July 2017
(q) Precedence Research "Cannabis Extract Market", Mar. 2020; includes
THC, CBD, CBG and other
Incannex
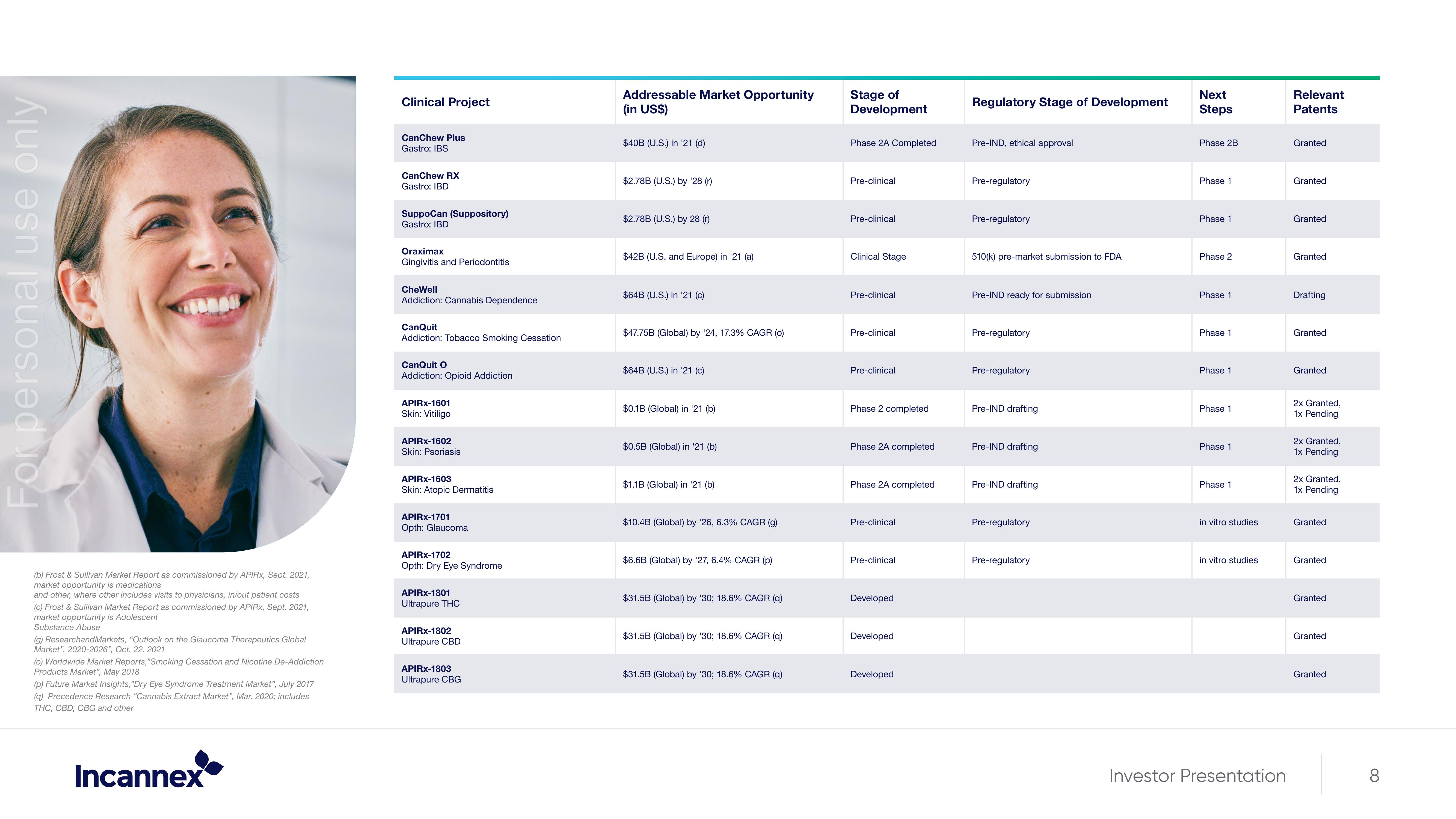
Clinical Project
CanChew Plus
Gastro: IBS
CanChew RX
Gastro: IBD
SuppoCan (Suppository)
Gastro: IBD
Oraximax
Gingivitis and Periodontitis
CheWell
Addiction: Cannabis Dependence
CanQuit
Addiction: Tobacco Smoking Cessation
CanQuit O
Addiction: Opioid Addiction
APIRX-1601
Skin: Vitiligo
APIRX-1602
Skin: Psoriasis
APIRX-1603
Skin: Atopic Dermatitis
APIRX-1701
Opth: Glaucoma
APIRX-1702
Opth: Dry Eye Syndrome
APIRX-1801
Ultrapure THC
APIRX-1802
Ultrapure CBD
APIRX-1803
Ultrapure CBG
Addressable Market Opportunity
(in US$)
$40B (U.S.) in '21 (d)
$2.78B (U.S.) by '28 (r)
$2.78B (U.S.) by 28 (r)
$42B (U.S. and Europe) in '21 (a)
$64B (U.S.) in '21 (c)
$47.75B (Global) by '24, 17.3 % CAGR (0)
$64B (U.S.) in '21 (c)
$0.1B (Global) in '21 (b)
$0.5B (Global) in '21 (b)
$1.1B (Global) in '21 (b)
$10.4B (Global) by '26, 6.3% CAGR (g)
$6.6B (Global) by '27, 6.4% CAGR (p)
$31.5B (Global) by '30; 18.6% CAGR (q)
$31.5B
'30; 18.6% CAGR (q)
$31.5B (Global) by '30; 18.6% CAGR (q)
Stage of
Development
Phase 2A Completed
Pre-clinical
Pre-clinical
Clinical Stage
Pre-clinical
Pre-clinical
Pre-clinical
Phase 2 completed
Phase 2A completed
Phase 2A completed
Pre-clinical
Pre-clinical
Developed
eloped
Developed
Regulatory Stage of Development
Pre-IND, ethical approval
Pre-regulatory
Pre-regulatory
510(k) pre-market submission to FDA
Pre-IND ready for submission
Pre-regulatory
Pre-regulatory
Pre-IND drafting
Pre-IND drafting
Pre-IND drafting
Pre-regulatory
Pre-regulatory
Next
Steps
Phase 2B
Phase 1
Phase 1
Phase 2
Phase 1
Phase 1
Phase 1
Phase 1
Phase 1
Phase 1
in vitro studies
in vitro studies
Investor Presentation
Relevant
Patents
Granted
Granted
Granted
Granted
Drafting
Granted
Granted
2x Granted,
1x Pending
2x Granted,
1x Pending
2x Granted,
1x Pending
Granted
Granted
Granted
Granted
Granted
8View entire presentation