Bluejay IPO Presentation Deck
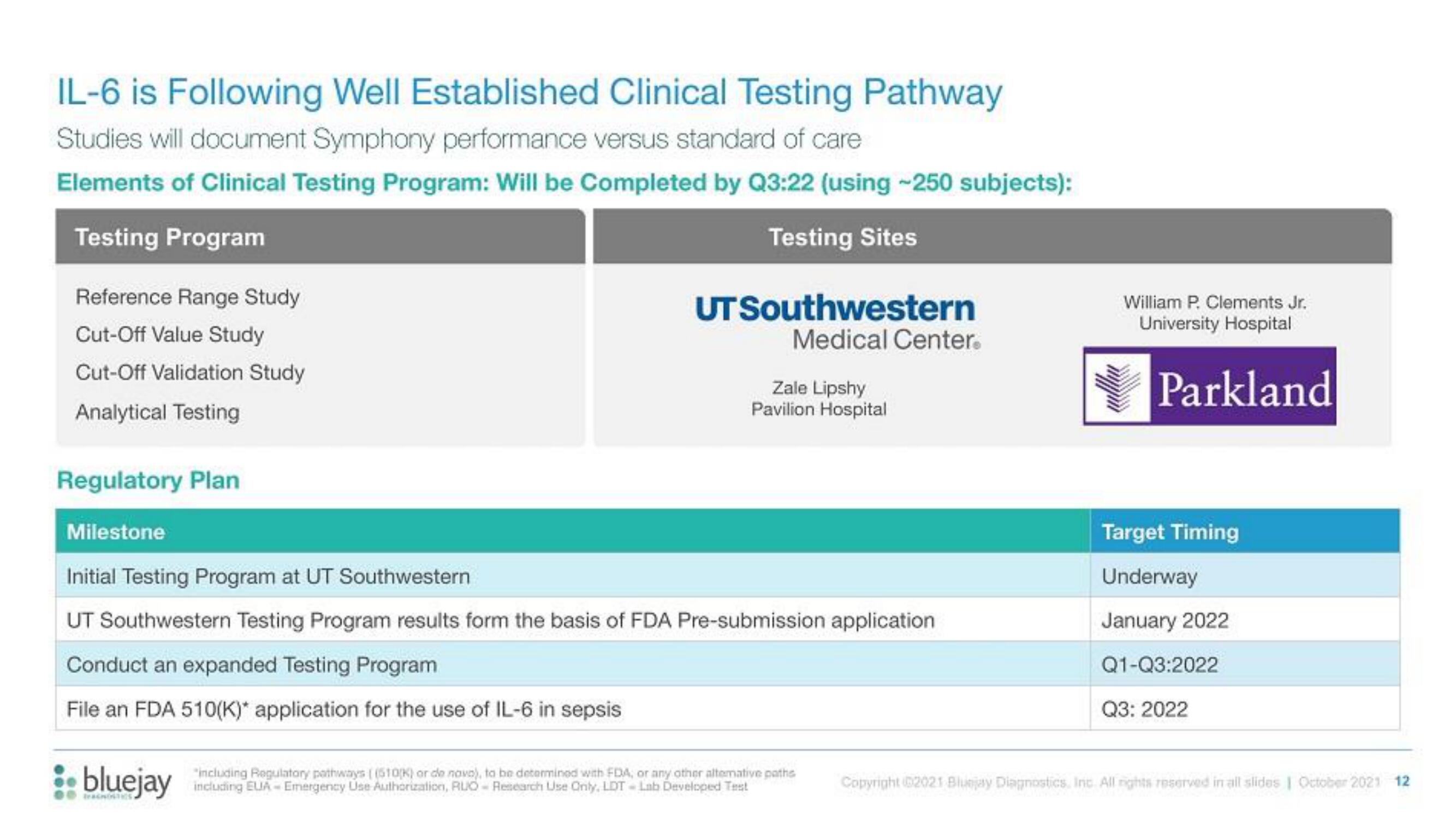
IL-6 is Following Well Established Clinical Testing Pathway
Studies will document Symphony performance versus standard of care
Elements of Clinical Testing Program: Will be Completed by Q3:22 (using -250 subjects):
Testing Program
Testing Sites
Reference Range Study
Cut-Off Value Study
Cut-Off Validation Study
Analytical Testing
Regulatory Plan
Milestone
UTSouthwestern
Medical Center®
Zale Lipshy
Pavilion Hospital
Initial Testing Program at UT Southwestern
UT Southwestern Testing Program results form the basis of FDA Pre-submission application
Conduct an expanded Testing Program
File an FDA 510(K)* application for the use of IL-6 in sepsis
"including Regulatory pathways ((5100K) or de nava), to be determined with FDA, or any other alterative paths
bluejay Authorization, - Only, LDT - Test
William P. Clements Jr.
University Hospital
Parkland
Target Timing
Underway
January 2022
Q1-Q3:2022
Q3: 2022
Copyright (02021 Blunjay Diagnostics, Inc. All rights reserved in all slides | October 2021 12View entire presentation