Context Therapeutics Investor Presentation Deck
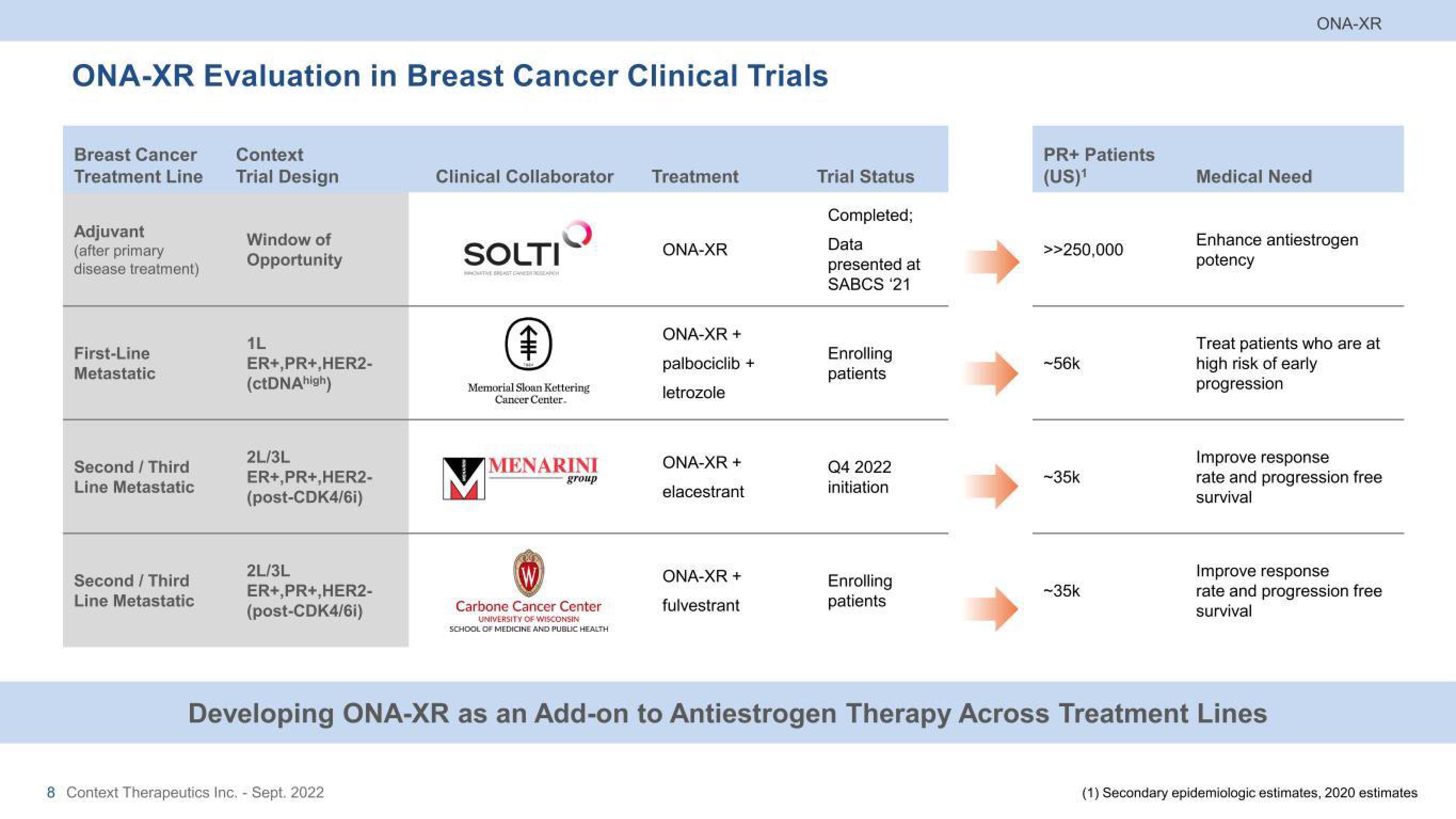
ONA-XR Evaluation in Breast Cancer Clinical Trials
Breast Cancer
Treatment Line
Adjuvant
(after primary
disease treatment)
First-Line
Metastatic
Second / Third
Line Metastatic
Second / Third
Line Metastatic
Context
Trial Design
Window of
Opportunity
1L
ER+,PR+,HER2-
(ctDNA high)
2L/3L
ER+,PR+,HER2-
(post-CDK4/6i)
2L/3L
ER+,PR+,HER2-
(post-CDK4/6i)
Clinical Collaborator Treatment
8 Context Therapeutics Inc. - Sept. 2022
SOLTI
←II+
M
#
THE
Memorial Sloan Kettering
Cancer Center.
MENARINI
group
(W)
Carbone Cancer Center
UNIVERSITY OF WISCONSIN
SCHOOL OF MEDICINE AND PUBLIC HEALTH
ONA-XR
ONA-XR +
palbociclib +
letrozole
ONA-XR +
elacestrant
ONA-XR +
fulvestrant
Trial Status
Completed;
Data
presented at
SABCS '21
Enrolling
patients
Q4 2022
initiation
Enrolling
patients
PR+ Patients
(US)¹
>>250,000
-56k
-35k
-35k
Medical Need
ONA-XR
Enhance antiestrogen
potency
Treat patients who are at
high risk of early
progression
Improve response
rate and progression free
survival
Developing ONA-XR as an Add-on to Antiestrogen Therapy Across Treatment Lines
Improve response
rate and progression free
survival
(1) Secondary epidemiologic estimates, 2020 estimatesView entire presentation