BioNTech Investor Day Presentation Deck
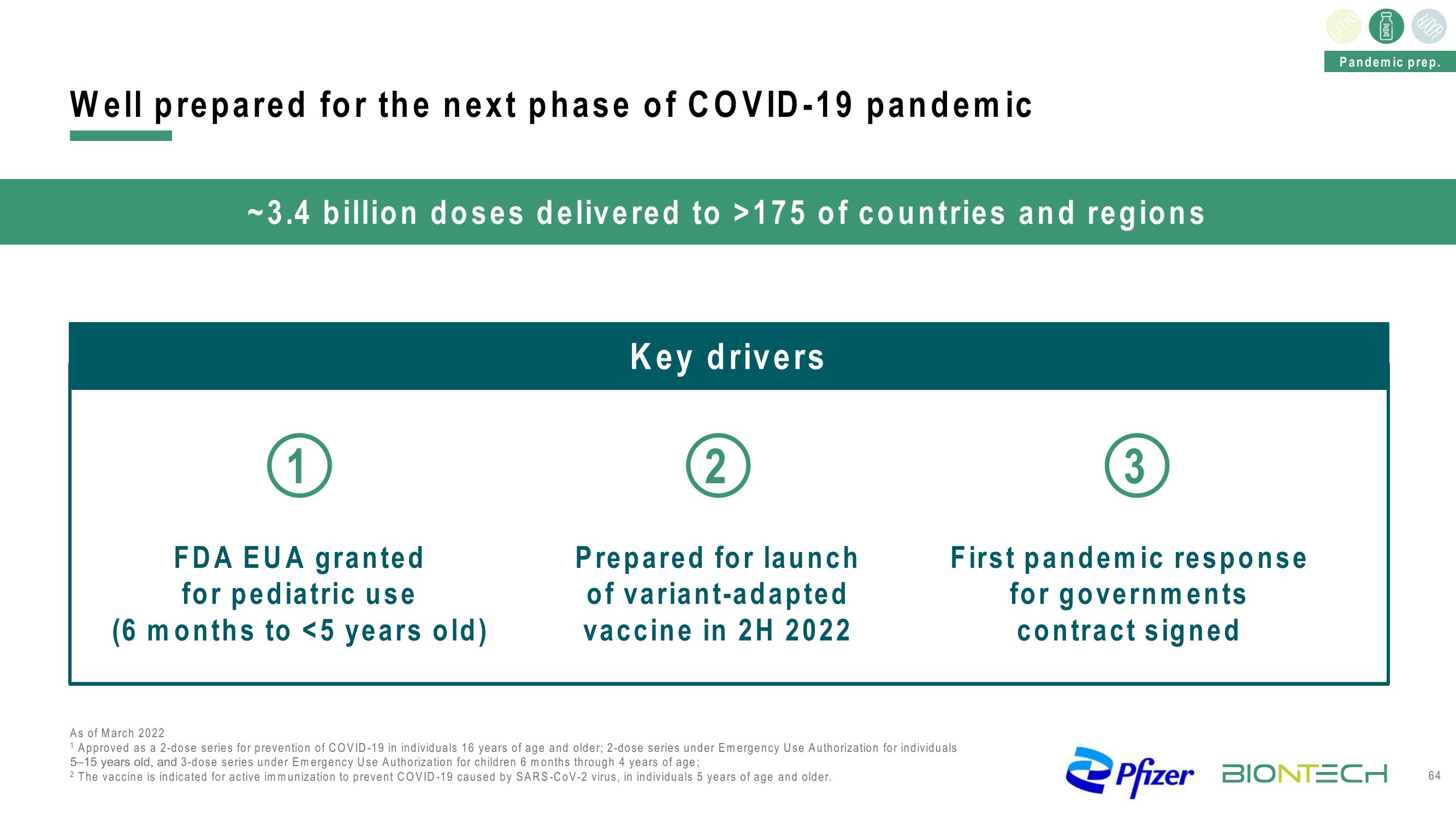
Well prepared for the next phase of COVID-19 pandemic
~3.4 billion doses delivered to >175 of countries and regions
1
FDA EUA granted
for pediatric use
(6 months to <5 years old)
Key drivers
2
Prepared for launch
of variant-adapted
vaccine in 2H 2022
3
First pandemic response
for governments
contract signed
As of March 2022
1 Approved as a 2-dose series for prevention of COVID-19 in individuals 16 years of age and older; 2-dose series under Emergency Use Authorization for individuals
5-15 years old, and 3-dose series under Emergency Use Authorization for children 6 months through 4 years of age;
2 The vaccine is indicated for active immunization to prevent COVID-19 caused by SARS-CoV-2 virus, in individuals 5 years of age and older.
CAR
Pandemic prep.
Pfizer BIONTECH
64View entire presentation