Immix Biopharma Investor Presentation Deck
NXC-201 Entering Large Markets At a Pivotal Moment
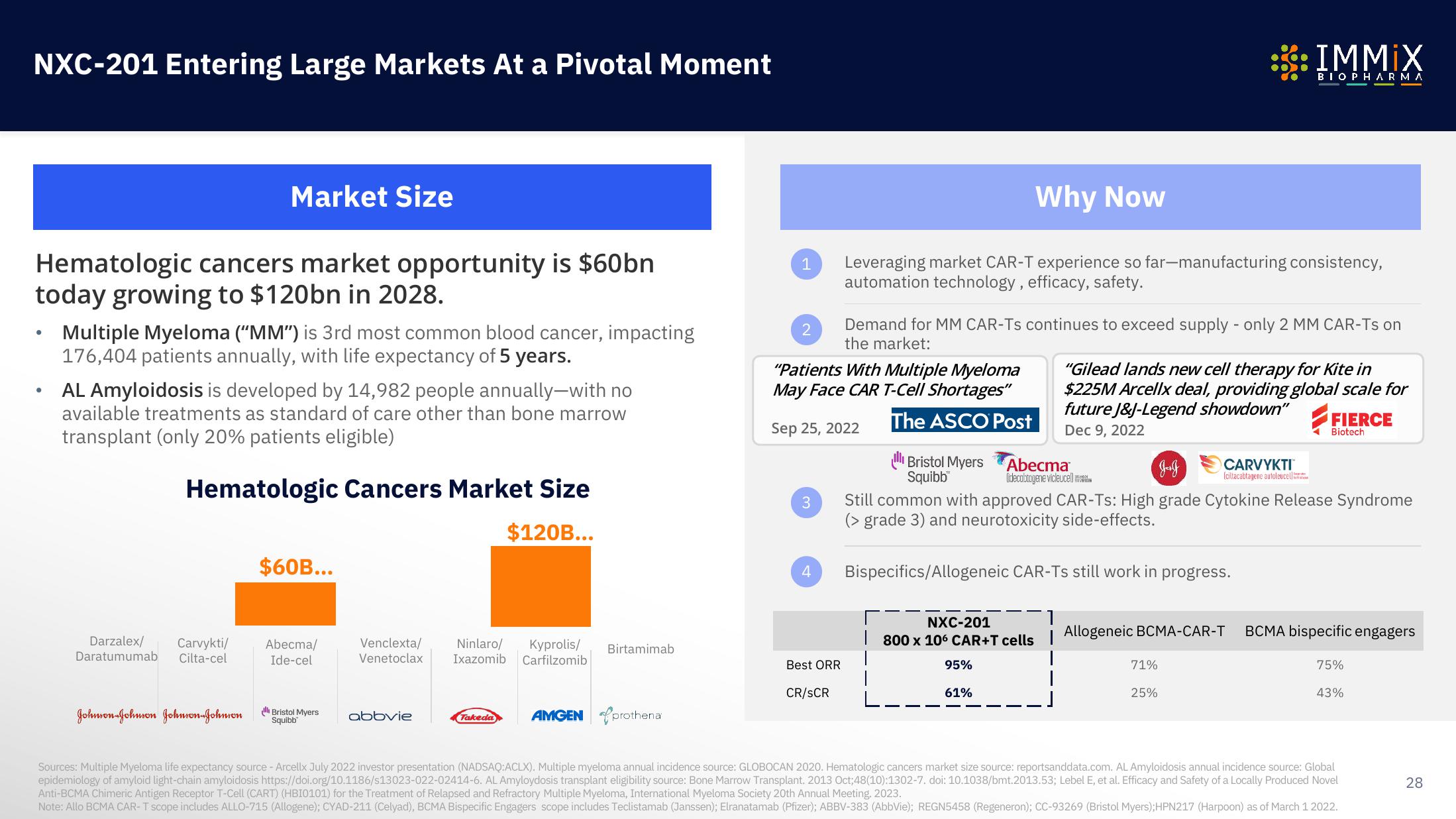
Hematologic cancers market opportunity is $60bn
today growing to $120bn in 2028.
●
Market Size
Multiple Myeloma ("MM") is 3rd most common blood cancer, impacting
176,404 patients annually, with life expectancy of 5 years.
AL Amyloidosis is developed by 14,982 people annually-with no
available treatments as standard of care other than bone marrow
transplant (only 20% patients eligible)
Hematologic Cancers Market Size
$120B...
Darzalex/ Carvykti/
Daratumumab Cilta-cel
Johnson & Johnson Johnson-Johnron
$60B...
Abecma/
Ide-cel
Bristol Myers
Squibb
Venclexta/
Venetoclax
abbvie
Ninlaro/ Kyprolis/
Ixazomib Carfilzomib
Birtamimab
Takeda AMGEN prothena
1
2
3
"Patients With Multiple Myeloma
May Face CAR T-Cell Shortages"
The ASCO Post
Sep 25, 2022
4
Best ORR
CR/SCR
Why Now
Leveraging market CAR-T experience so far-manufacturing consistency,
automation technology, efficacy, safety.
Demand for MM CAR-Ts continues to exceed supply - only 2 MM CAR-Ts on
the market:
Ill Bristol Myers
Squibb
Abecma
decobtogene vicleucell .
NXC-201
800 x 106 CAR+T cells
95%
"Gilead lands new cell therapy for Kite in
$225M Arcellx deal, providing global scale for
future J&J-Legend showdown"
Dec 9, 2022
Bispecifics/Allogeneic CAR-Ts still work in progress.
1
61%
Still common with approved CAR-Ts: High grade Cytokine Release Syndrome
grade 3) and neurotoxicity side-effects.
●●●
IMMIX
S BIOPHARMA
CARVYKTI
(ciltacabtagene autoleucell
Allogeneic BCMA-CAR-T
71%
25%
FIERCE
Biotech
BCMA bispecific engagers
75%
43%
Sources: Multiple Myeloma life expectancy source - Arcellx July 2022 investor presentation (NADSAQ:ACLX). Multiple myeloma annual incidence source: GLOBOCAN 2020. Hematologic cancers market size source: reportsanddata.com. AL Amyloidosis annual incidence source: Global
epidemiology of amyloid light-chain amyloidosis https://doi.org/10.1186/s13023-022-02414-6. AL Amyloydosis transplant eligibility source: Bone Marrow Transplant. 2013 Oct;48(10):1302-7. doi: 10.1038/bmt.2013.53; Lebel E, et al. Efficacy and Safety of a Locally Produced Novel
Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting, 2023.
Note: Allo BCMA CAR-T scope includes ALLO-715 (Allogene); CYAD-211 (Celyad), BCMA Bispecific Engagers scope includes Teclistamab (Janssen); Elranatamab (Pfizer); ABBV-383 (AbbVie); REGN5458 (Regeneron); CC-93269 (Bristol Myers); HPN217 (Harpoon) as of March 1 2022.
28View entire presentation