OmniAb SPAC Presentation Deck
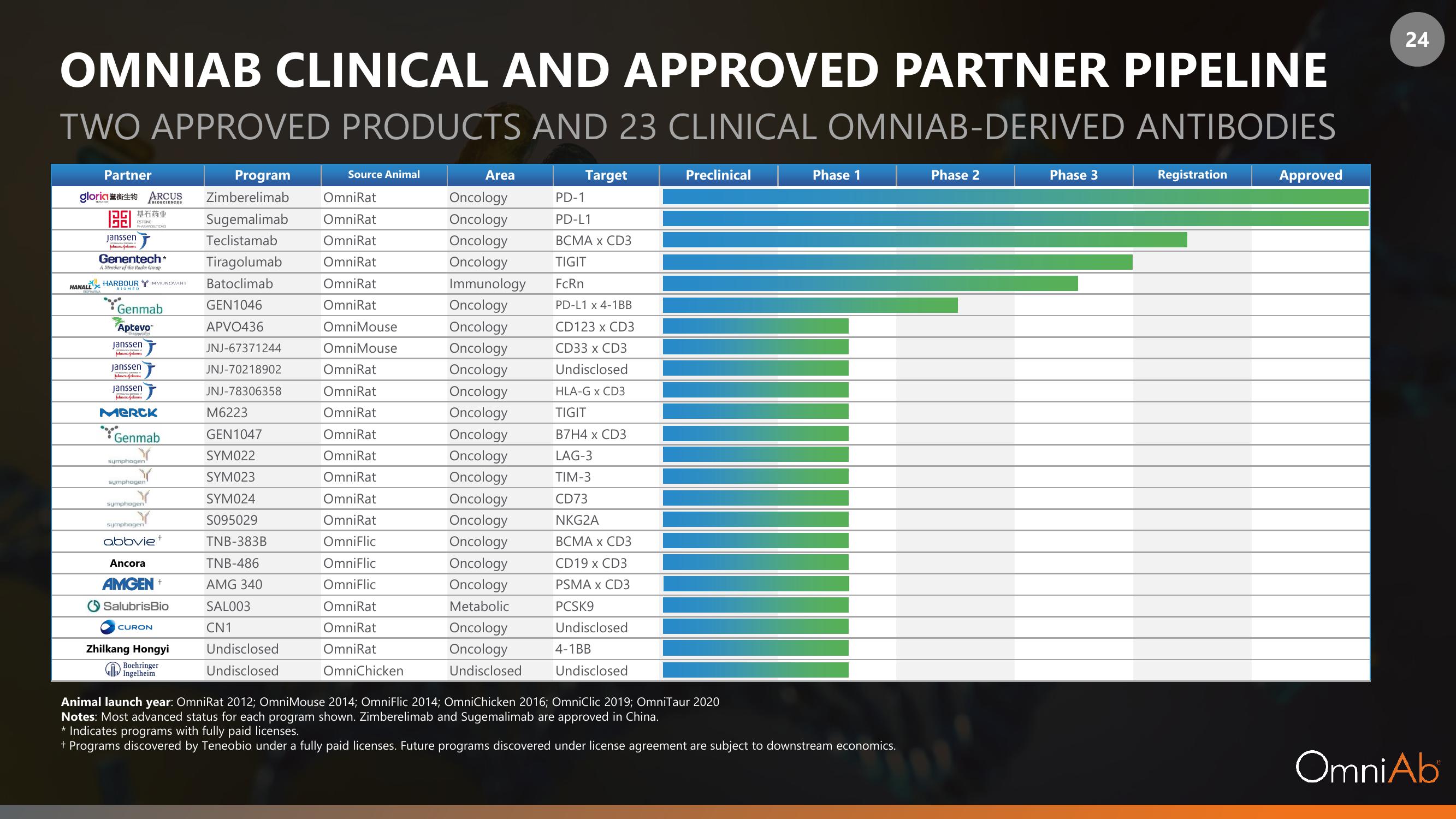
OMNIAB CLINICAL AND APPROVED PARTNER PIPELINE
TWO APPROVED PRODUCTS AND 23 CLINICAL OMNIAB-DERIVED ANTIBODIES
Partner
gloria 誉衡生物 ARCUS
基石药业
BIOSCIENCES
CSTONE
PHARMACEUTICAS
Janssen
Johan Jokureu
Genentech*
A Member of the Bocke Group
HANALL HARBOUR IMMUNOVANT
Genmab
Aptevo
Janssen
John Acken
Janssen
Janssen
Johna Jakuen
Merck
Genmab
symphogen
symphogen
symphogen!
symphagen
abbviet
Ancora
AMGEN +
SalubrisBio
O CURON
Zhilkang Hongyi
Boehringer
Ingelheim
Program
Zimberelimab OmniRat
Sugemalimab
OmniRat
OmniRat
Teclistamab
OmniRat
OmniRat
OmniRat
OmniMouse
OmniMouse
OmniRat
OmniRat
OmniRat
OmniRat
OmniRat
OmniRat
OmniRat
OmniRat
OmniFlic
OmniFlic
OmniFlic
OmniRat
OmniRat
OmniRat
OmniChicken
Tiragolumab
Batoclimab
GEN1046
APVO436
JNJ-67371244
JNJ-70218902
JNJ-78306358
M6223
GEN1047
SYM022
SYM023
SYM024
S095029
TNB-383B
TNB-486
AMG 340
SAL003
CN1
Source Animal
Undisclosed
Undisclosed
Area
Oncology
Oncology
Oncology
Oncology
Immunology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Oncology
Metabolic
Oncology
Oncology
Undisclosed
Target
PD-1
PD-L1
BCMA x CD3
TIGIT
FcRn
PD-L1 x 4-1BB
CD123 x CD3
CD33 x CD3
Undisclosed
HLA-G x CD3
TIGIT
B7H4 x CD3
LAG-3
TIM-3
CD73
NKG2A
BCMA x CD3
CD19 x CD3
PSMA x CD3
PCSK9
Undisclosed
4-1BB
Undisclosed
Preclinical
Animal launch year: OmniRat 2012; OmniMouse 2014; OmniFlic 2014; OmniChicken 2016; OmniClic 2019; OmniTaur 2020
Notes: Most advanced status for each program shown. Zimberelimab and Sugemalimab are approved in China.
Phase 1
* Indicates programs with fully paid licenses.
+ Programs discovered by Teneobio under a fully paid licenses. Future programs discovered under license agreement are subject to downstream economics.
Phase 2
Phase 3
Registration
Approved
24
OmniAbView entire presentation