AstraZeneca Results Presentation Deck
Tagrisso and Imfinzi
Global growth boosted by Europe, Rest of World
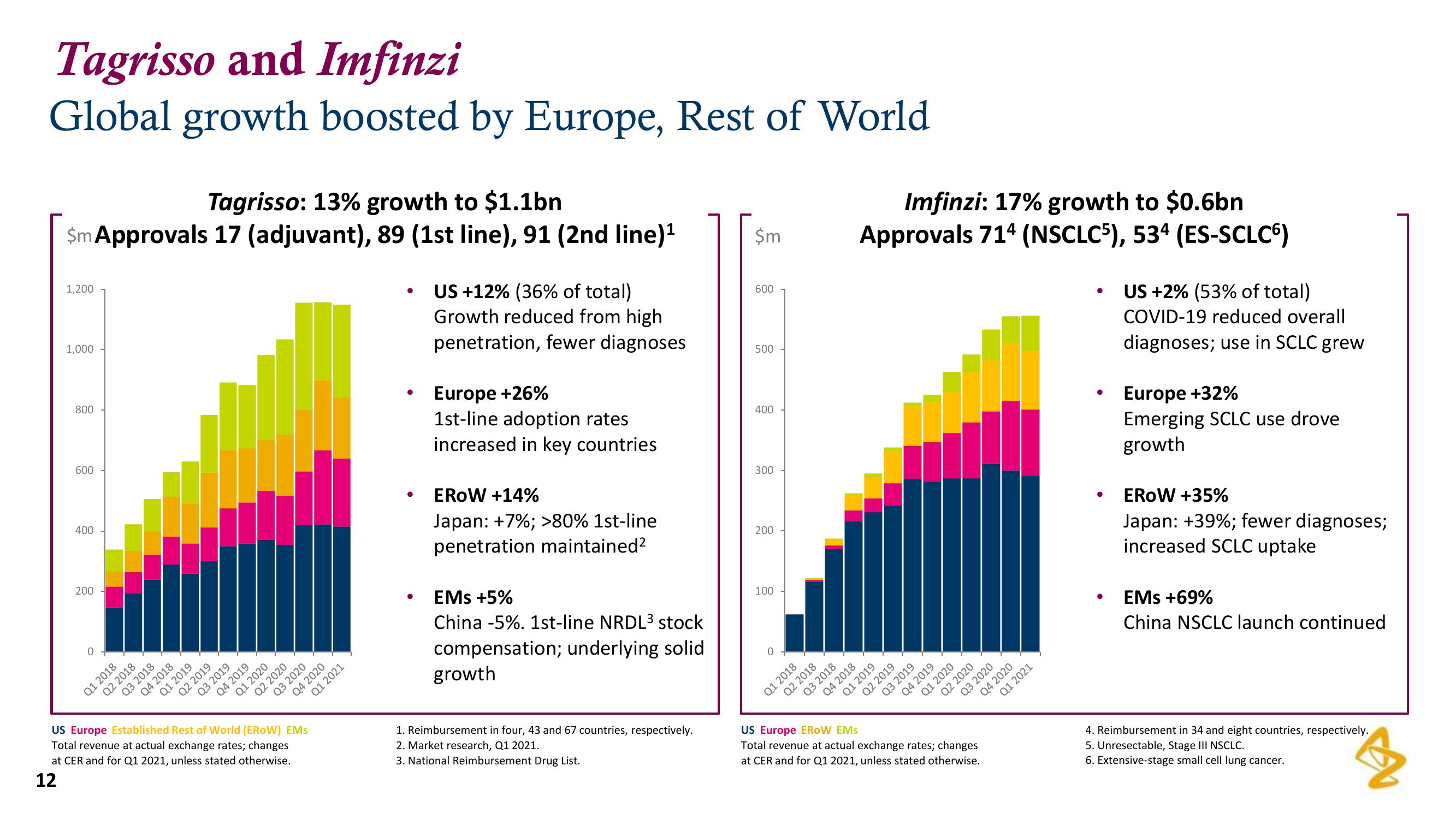
Tagrisso: 13% growth to $1.1bn
$m Approvals 17 (adjuvant), 89 (1st line), 91 (2nd line)¹
1,200
1,000
800
600
400
200
0+
Q1 2018
Q2 2018
Q3 2018
Q4 2018
Q1 2019
Q2 2019
Q3 2019
Q4 2019
Q1 2020
Q2 2020
Q3 2020
US Europe Established Rest of World (EROW) EMS
Total revenue at actual exchange rates; changes
at CER and for Q1 2021, unless stated otherwise.
12
Q4 2020
Q1 2021
●
●
●
●
US +12% (36% of total)
Growth reduced from high
penetration, fewer diagnoses.
Europe +26%
1st-line adoption rates
increased in key countries
EROW +14%
Japan: +7%; >80% 1st-line
penetration maintained²
EMs +5%
China -5%. 1st-line NRDL³ stock
compensation; underlying solid
growth
1. Reimbursement in four, 43 and 67 countries, respectively.
2. Market research, Q1 2021.
3. National Reimbursement Drug List.
$m
600
500
400
300
200
100
Q1 2018
Q2 2018
Q3
Imfinzi: 17% growth to $0.6bn
Approvals 714 (NSCLC5), 534 (ES-SCLC6)
2018
Q4 2018
Q1 2019
Q2 2019
Q3 2019
Q4
2019
Q1 2020
Q2 2020
US Europe
EROW EMS
Total revenue at actual
rates; changes
exchange
at CER and for Q1 2021, unless stated otherwise.
Q3 2020
Q4 2020
Q1 2021
●
●
●
US +2% (53% of total)
COVID-19 reduced overall
diagnoses; use in SCLC grew
Europe +32%
Emerging SCLC use drove
growth
EROW +35%
Japan: +39%; fewer diagnoses;
increased SCLC uptake
EMS +69%
China NSCLC launch continued
4. Reimbursement in 34 and eight countries, respectively.
5. Unresectable, Stage III NSCLC.
6. Extensive-stage small cell lung cancer.
3View entire presentation