ATAI Investor Presentation Deck
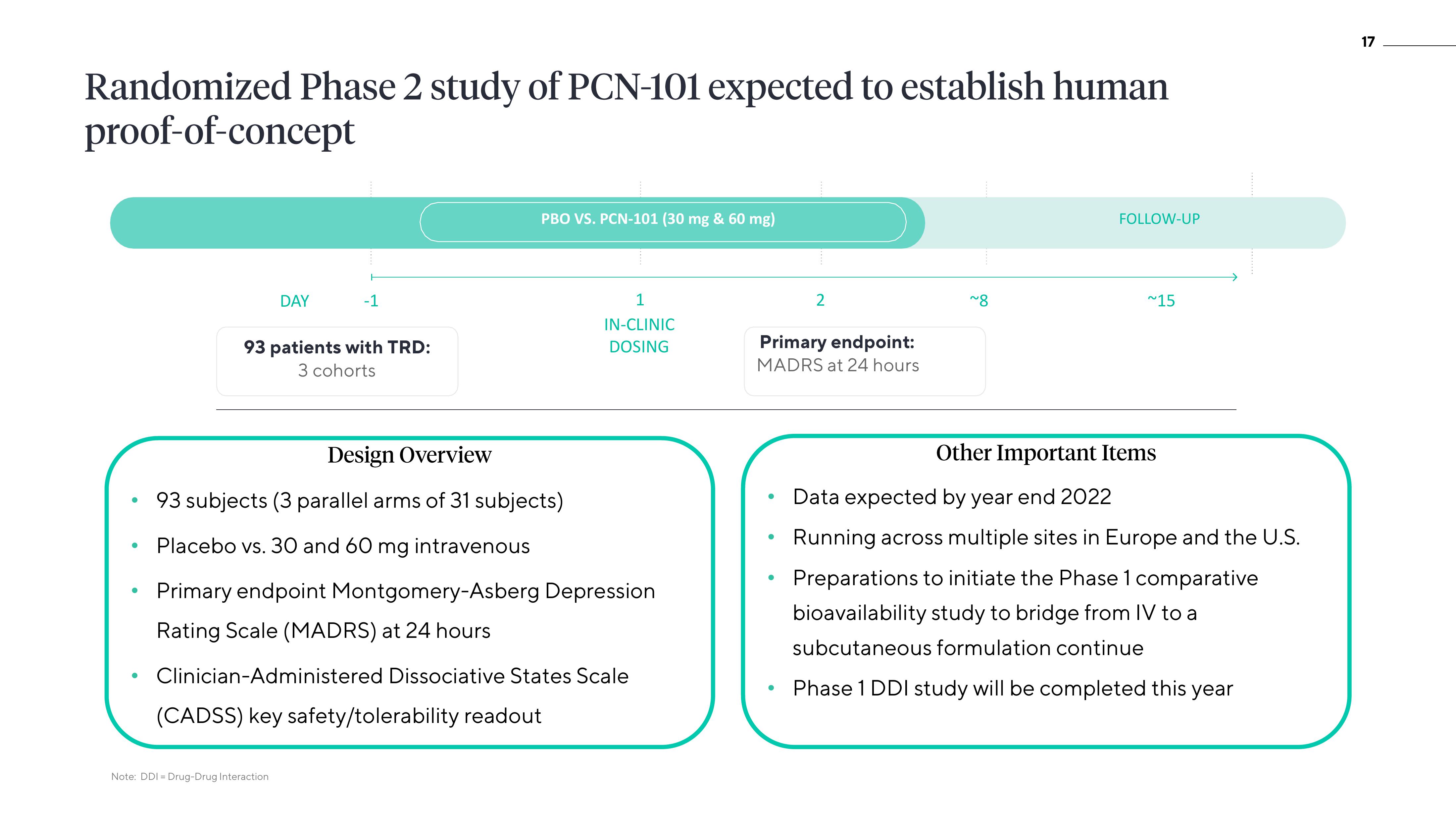
Randomized Phase 2 study of PCN-101 expected to establish human
proof-of-concept
DAY
-1
93 patients with TRD:
3 cohorts
PBO VS. PCN-101 (30 mg & 60 mg)
Design Overview
93 subjects (3 parallel arms of 31 subjects)
Placebo vs. 30 and 60 mg intravenous
Note: DDI - Drug-Drug Interaction
1
IN-CLINIC
DOSING
Primary endpoint Montgomery-Asberg Depression
Rating Scale (MADRS) at 24 hours
Clinician-Administered Dissociative States Scale
(CADSS) key safety/tolerability readout
Primary endpoint:
MADRS at 24 hours
●
2
•
~8
FOLLOW-UP
~15
Other Important Items
Data expected by year end 2022
Running across multiple sites in Europe and the U.S.
Preparations to initiate the Phase 1 comparative
bioavailability study to bridge from IV to a
subcutaneous formulation continue
Phase 1 DDI study will be completed this year
17View entire presentation