Immix Biopharma Investor Presentation Deck
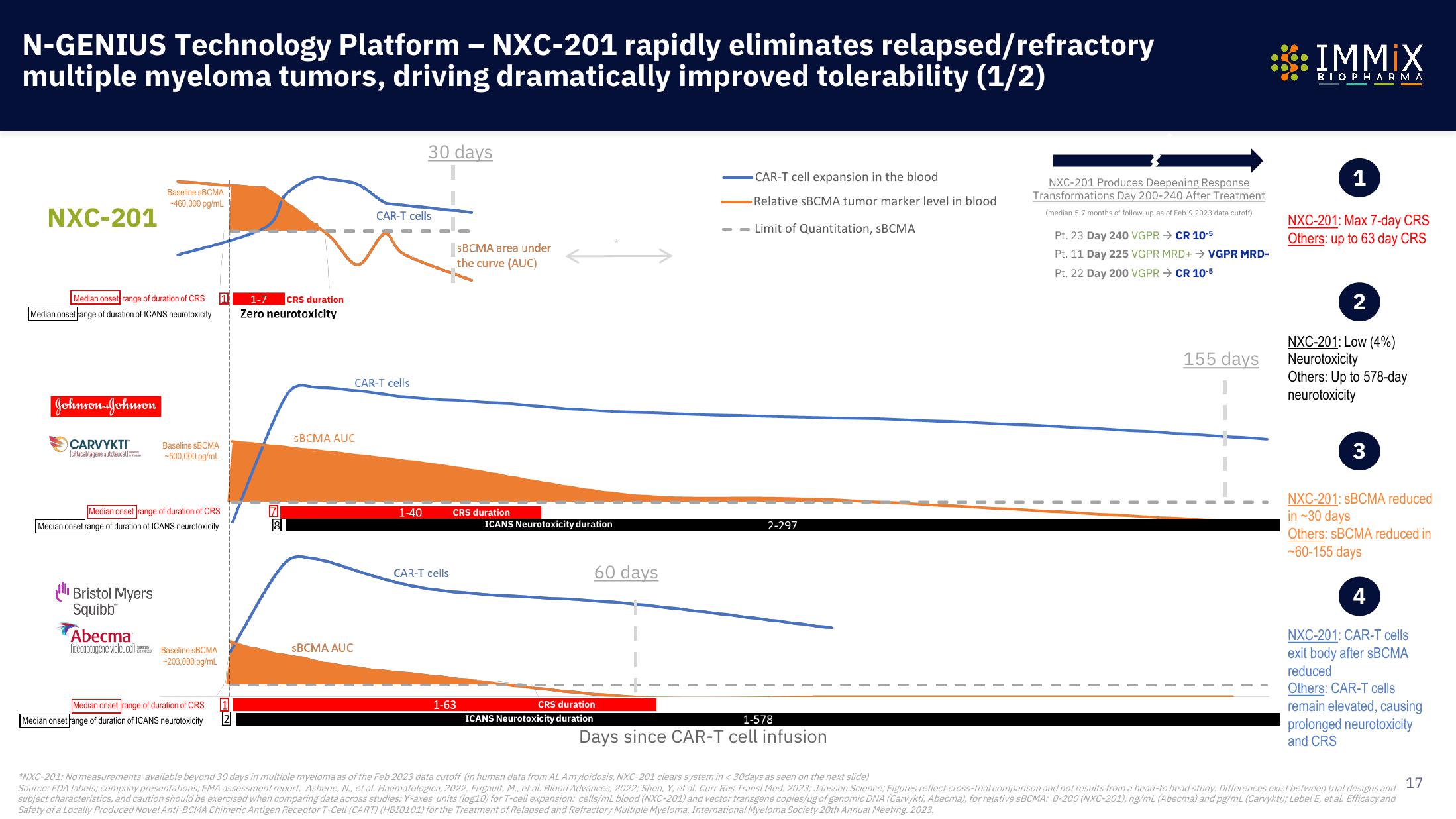
N-GENIUS Technology Platform - NXC-201 rapidly eliminates relapsed/refractory
multiple myeloma tumors, driving dramatically improved tolerability (1/2)
NXC-201
Median onset range of duration of CRS
Median onset range of duration of ICANS neurotoxicity
Johnson & Johnson
CARVYKTI™
(citacabtagene autoleucell
Baseline SBCMA
-460,000 pg/mL
Ill Bristol Myers
Squibb™
Median onset range of duration of CRS
Median onset range of duration of ICANS neurotoxicity
Abecma
(idecabtagene vicleuce)
Baseline SBCMA
-500,000 pg/mL
Baseline SBCMA
-203,000 pg/mL
Median onset range of duration of CRS
Median onset range of duration of ICANS neurotoxicity
1
1
2
1-7 CRS duration
Zero neurotoxicity
sBCMA AUC
sBCMA AUC
CAR-T cells
CAR-T cells
30 days
1-40
CAR-T cells
SBCMA area under
the curve (AUC)
CRS duration
1-63
ICANS Neurotoxicity duration
60 days
CRS duration
ICANS Neurotoxicity duration
CAR-T cell expansion in the blood
-Relative SBCMA tumor marker level in blood
--Limit of Quantitation, SBCMA
2-297
1-578
Days since CAR-T cell infusion
NXC-201 Produces Deepening Response
Transformations Day 200-240 After Treatment
(median 5.7 months of follow-up as of Feb 9 2023 data cutoff)
Pt. 23 Day 240 VGPR → CR 10-5
Pt. 11 Day 225 VGPR MRD+ → VGPR MRD-
Pt. 22 Day 200 VGPR CR 10-5
155 days
●●●
S
IMMİX
BIOPHARMA
1
NXC-201: Max 7-day CRS
Others: up to 63 day CRS
2
NXC-201: Low (4%)
Neurotoxicity
Others: Up to 578-day
neurotoxicity
3
NXC-201: SBCMA reduced
in ~30 days
Others: SBCMA reduced in
-60-155 days
4
NXC-201: CAR-T cells
exit body after sBCMA
reduced
Others: CAR-T cells
remain elevated, causing
prolonged neurotoxicity
and CRS
*NXC-201: No measurements available beyond 30 days in multiple myeloma as of the Feb 2023 data cutoff (in human data from AL Amyloidosis, NXC-201 clears system in < 30days as seen on the next slide)
17
Source: FDA labels; company presentations; EMA assessment report; Asherie, N., et al. Haematologica, 2022. Frigault, M., et al. Blood Advances, 2022; Shen, Y, et al. Curr Res Transl Med. 2023; Janssen Science; Figures reflect cross-trial comparison and not results from a head-to head study. Differences exist between trial designs and
subject characteristics, and caution should be exercised when comparing data across studies; Y-axes units (log10) for T-cell expansion: cells/mL blood (NXC-201) and vector transgene copies/ug of genomic DNA (Carvykti, Abecma), for relative SBCMA: 0-200 (NXC-201), ng/mL (Abecma) and pg/mL (Carvykti); Lebel E, et al. Efficacy and
Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting. 2023.View entire presentation