Dare Bioscience Investor Presentation Deck
Sildenafil Cream, 3.6% - Phase 1 and Phase 2a Study Results
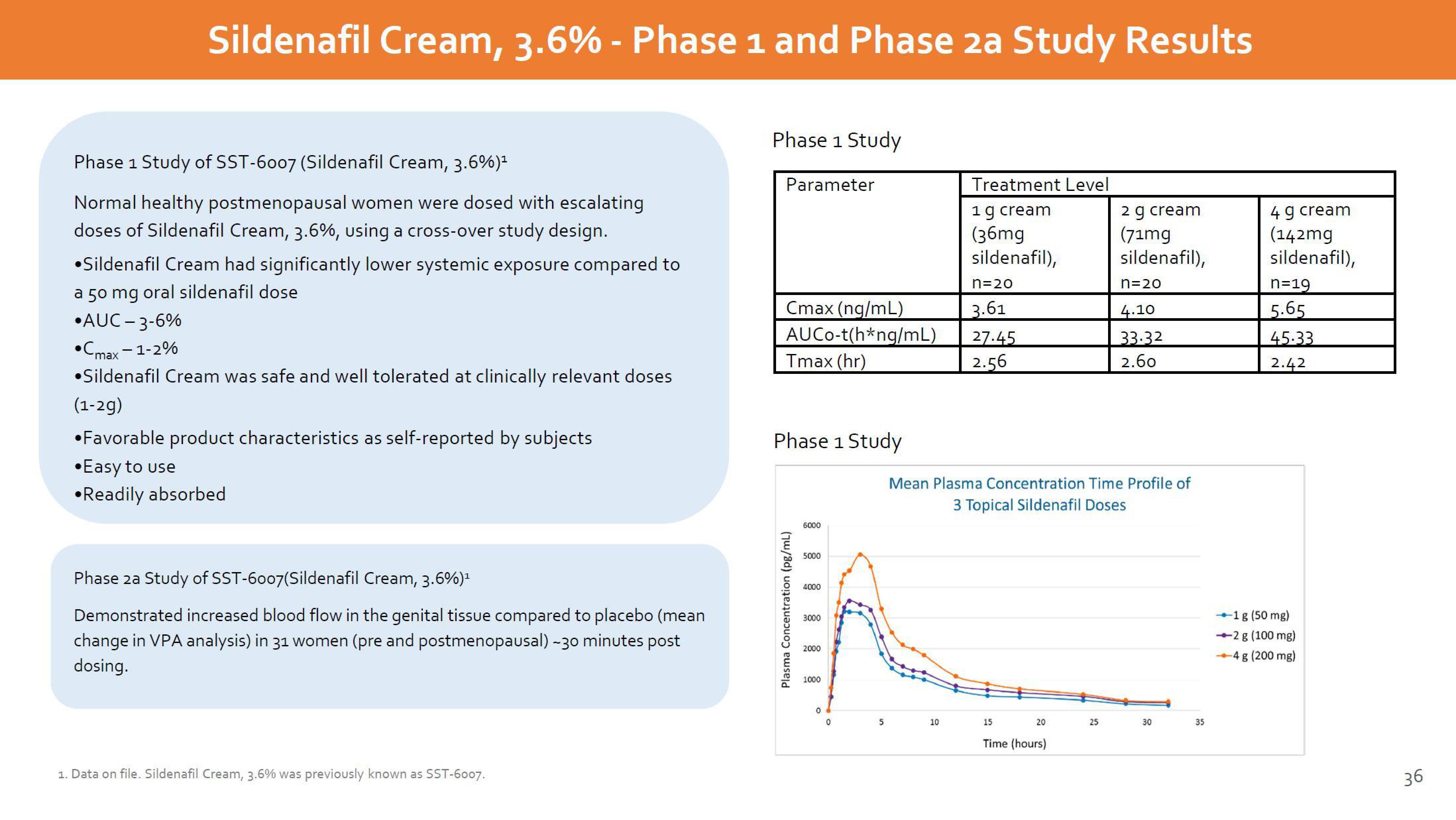
Phase 1 Study of SST-6007 (Sildenafil Cream, 3.6%)¹
Normal healthy postmenopausal women were dosed with escalating
doses of Sildenafil Cream, 3.6%, using a cross-over study design.
•Sildenafil Cream had significantly lower systemic exposure compared to
a 50 mg oral sildenafil dose
•AUC -3-6%
•Cmax-1-2%
•Sildenafil Cream was safe and well tolerated at clinically relevant doses
(1-2g)
•Favorable product characteristics as self-reported by subjects
• Easy to use
Readily absorbed
Phase 2a Study of SST-6007(Sildenafil Cream, 3.6%)¹
Demonstrated increased blood flow in the genital tissue compared to placebo (mean
change in VPA analysis) in 31 women (pre and postmenopausal) -30 minutes post
dosing.
1. Data on file. Sildenafil Cream, 3.6% was previously known as SST-6007.
Phase 1 Study
Parameter
Cmax (ng/mL)
AUCo-t(h*ng/mL)
Tmax (hr)
Phase 1 Study
Plasma Concentration (pg/mL)
6000
5000
4000
3000
2000
1000
0
0
5
Treatment Level
1 g cream
(36mg
sildenafil),
10
n=20
3.61
27.45
2.56
Mean Plasma Concentration Time Profile of
3 Topical Sildenafil Doses
15
20
Time (hours)
2 g cream
(71mg
sildenafil),
25
n=20
4.10
33.32
2.60
30
35
4 g cream
(142mg
sildenafil),
n=19
5.65
45.33
2.42
-1 g (50 mg)
-2 g (100 mg)
-4 g (200 mg)
36View entire presentation