argenx SE Investor Day Presentation Deck
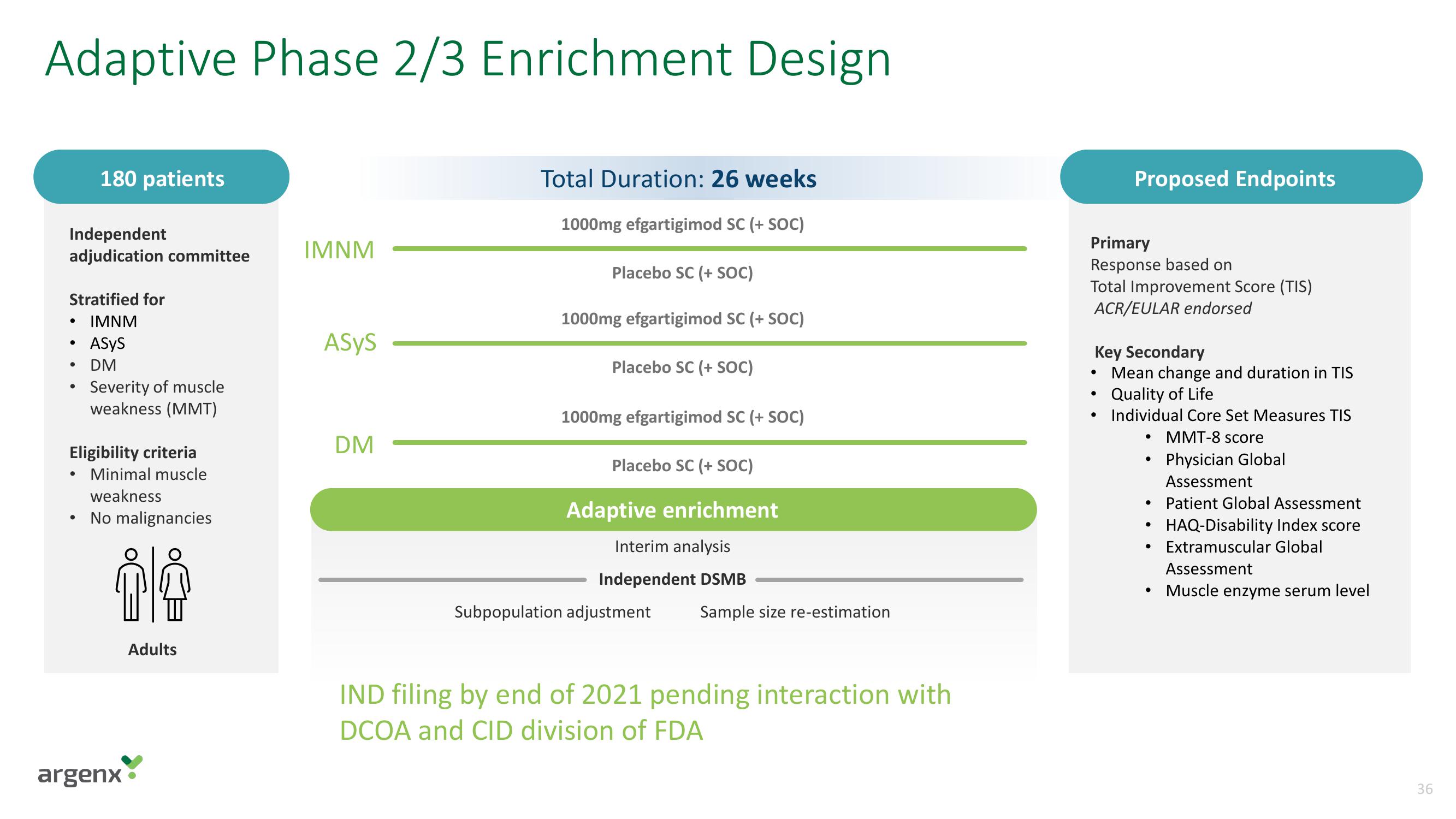
Adaptive Phase 2/3 Enrichment Design
Independent
adjudication committee
Stratified for
IMNM
ASYS
DM
●
●
●
180 patients
●
●
Severity of muscle
weakness (MMT)
Eligibility criteria
• Minimal muscle
weakness
No malignancies
ili
argenx
Adults
IMNM
ASYS
DM
Total Duration: 26 weeks
1000mg efgartigimod SC (+ SOC)
Placebo SC (+ SOC)
1000mg efgartigimod SC (+ SOC)
Placebo SC (+ SOC)
1000mg efgartigimod SC (+ SOC)
Placebo SC (+ SOC)
Adaptive enrichment
Interim analysis
Independent DSMB
Subpopulation adjustment
Sample size re-estimation
IND filing by end of 2021 pending interaction with
DCOA and CID division of FDA
Primary
Response based on
Total Improvement Score (TIS)
ACR/EULAR endorsed
Key Secondary
Mean change and duration in TIS
Quality of Life
Individual Core Set Measures TIS
●
Proposed Endpoints
●
●
●
●
●
●
●
●
MMT-8 score
Physician Global
Assessment
Patient Global Assessment
HAQ-Disability Index score
Extramuscular Global
Assessment
Muscle enzyme serum level
36View entire presentation