AstraZeneca Results Presentation Deck
CAR copies/ug gDNA
1x106
1x105
1x104
1x10³-
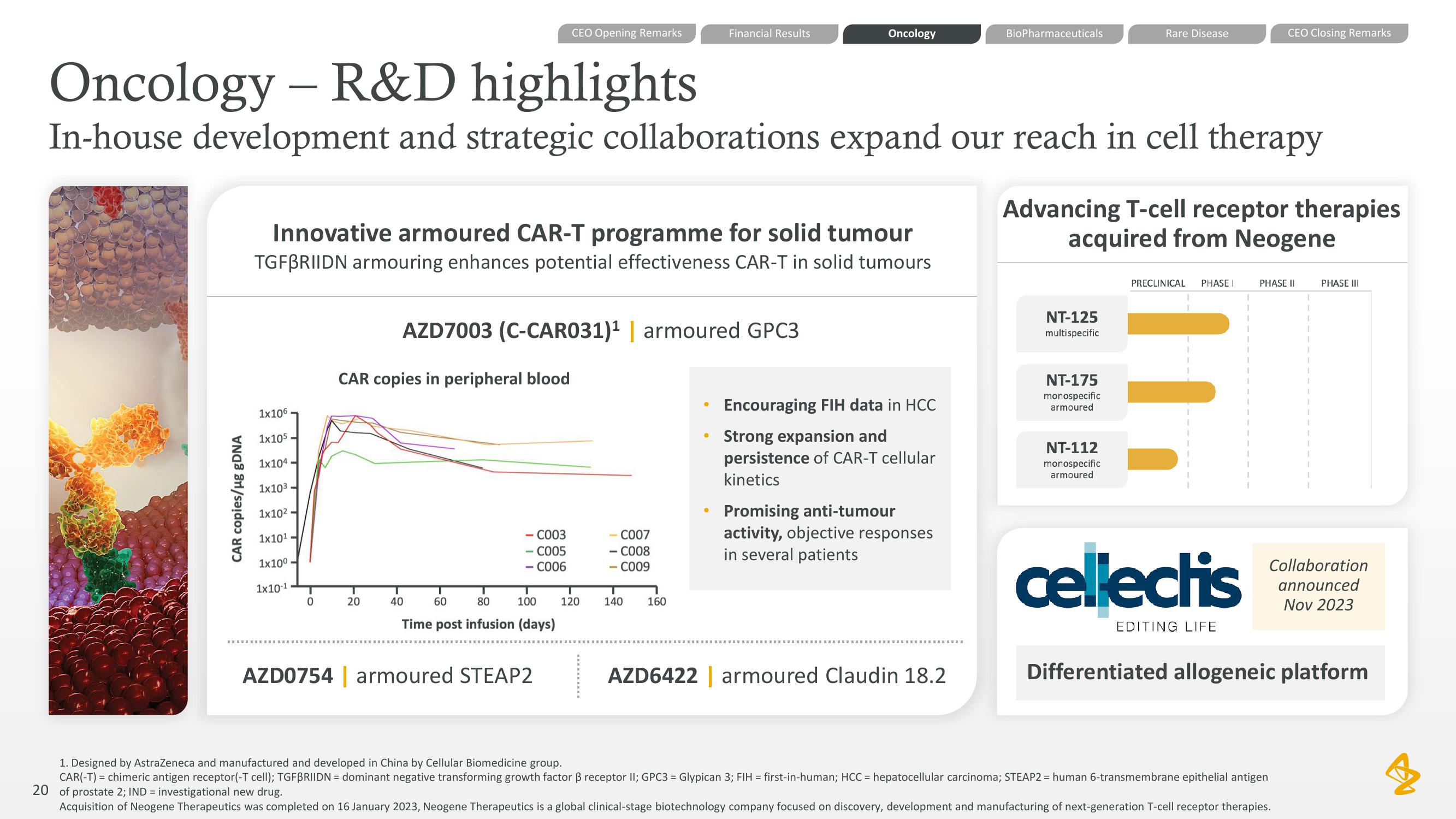
Innovative armoured CAR-T programme for solid tumour
TGFBRIIDN armouring enhances potential effectiveness CAR-T in solid tumours
1x10²
1x10¹
1x10⁰
1x10-1
Oncology - R&D highlights
In-house development and strategic collaborations expand our reach in cell therapy
0
CAR copies in peripheral blood
20
AZD7003 (C-CAR031)¹ | armoured GPC3
40
CEO Opening Remarks
-C003
-C005
-C006
60
80
100
Time post infusion (days)
AZD0754 armoured STEAP2
120
Financial Results
-C007
-C008
- C009
140
Oncology
160
Encouraging FIH data in HCC
Strong expansion and
persistence of CAR-T cellular
kinetics
Promising anti-tumour
activity, objective responses
in several patients
BioPharmaceuticals
AZD6422 | armoured Claudin 18.2
NT-125
multispecific
Rare Disease
Advancing T-cell receptor therapies
acquired from Neogene
NT-175
monospecific
armoured
NT-112
monospecific
armoured
PRECLINICAL PHASE I
CEO Closing Remarks
cellectis
EDITING LIFE
PHASE II
PHASE III
Collaboration
announced
Νον 2023
1. Designed by AstraZeneca and manufactured and developed in China by Cellular Biomedicine group.
CAR(-T) = chimeric antigen receptor(-T cell); TGFBRIIDN = dominant negative transforming growth factor ß receptor II; GPC3 = Glypican 3; FIH = first-in-human; HCC = hepatocellular carcinoma; STEAP2 = human 6-transmembrane epithelial antigen
20 of prostate 2; IND = investigational new drug.
Acquisition of Neogene Therapeutics was completed on 16 January 2023, Neogene Therapeutics is a global clinical-stage biotechnology company focused on discovery, development and manufacturing of next-generation T-cell receptor therapies.
Differentiated allogeneic platform
BView entire presentation