Kymera Investor Day Presentation Deck
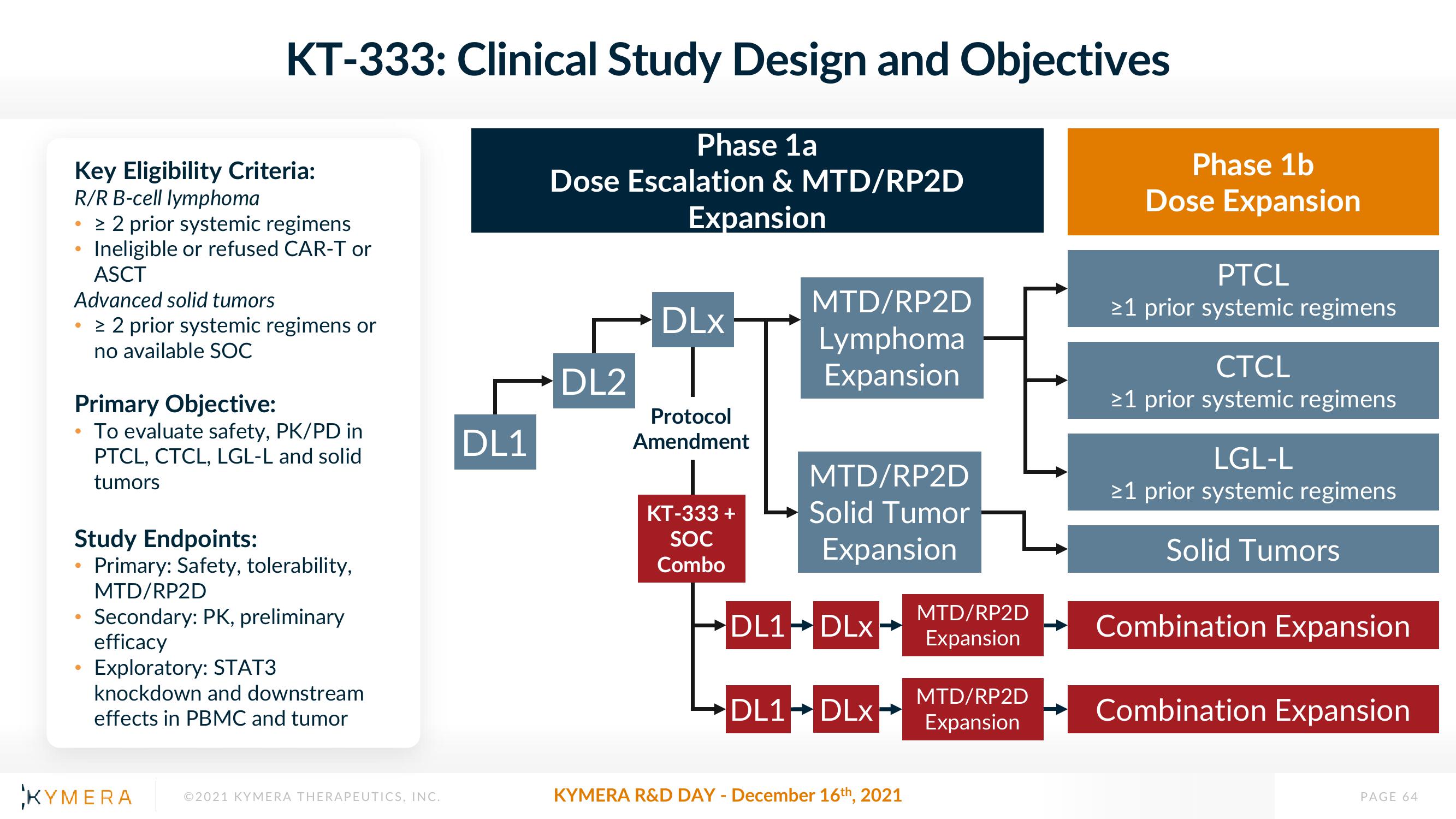
Key Eligibility Criteria:
R/R B-cell lymphoma
•> 2 prior systemic regimens
Ineligible or refused CAR-T or
ASCT
Advanced solid tumors
> 2 prior systemic regimens or
no available SOC
●
●
KT-333: Clinical Study Design and Objectives
Primary Objective:
To evaluate safety, PK/PD in
PTCL, CTCL, LGL-L and solid
tumors
●
Study Endpoints:
Primary: Safety, tolerability,
●
●
MTD/RP2D
Secondary: PK, preliminary
efficacy
Exploratory: STAT3
knockdown and downstream
effects in PBMC and tumor
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
DL1
Phase 1a
Dose Escalation & MTD/RP2D
Expansion
DL2
DLx
Protocol
Amendment
KT-333 +
SOC
Combo
MTD/RP2D
Lymphoma
Expansion
MTD/RP2D
Solid Tumor
Expansion
DL1-DLX
DL1 DLx→
KYMERA R&D DAY - December 16th, 2021
MTD/RP2D
Expansion
MTD/RP2D
Expansion
Phase 1b
Dose Expansion
PTCL
≥1 prior systemic regimens
CTCL
≥1 prior systemic regimens
LGL-L
≥1 prior systemic regimens
Solid Tumors
Combination Expansion
Combination Expansion
PAGE 64View entire presentation