BioNTech Investor Day Presentation Deck
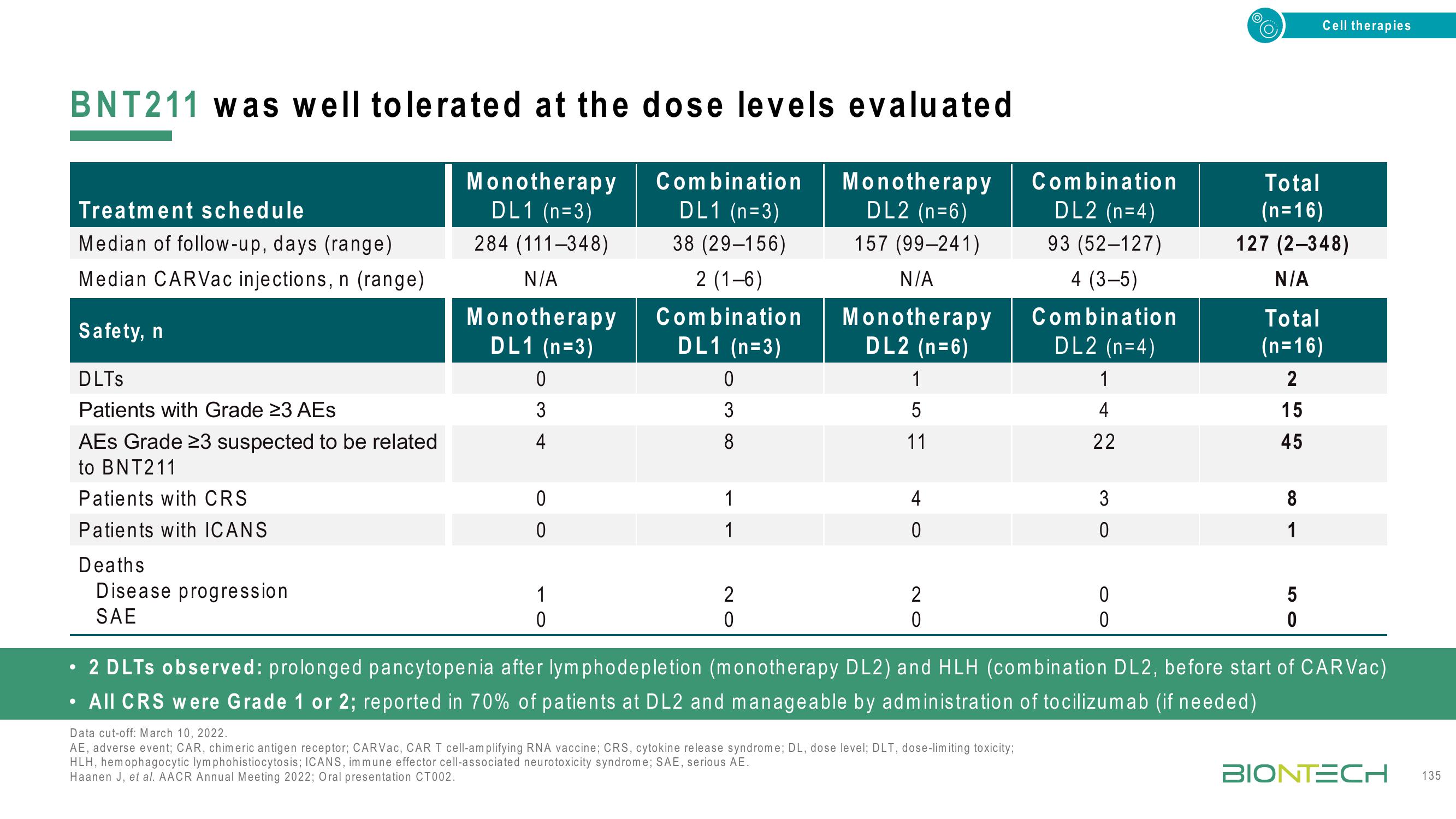
BNT211 was well tolerated at the dose levels evaluated
Monotherapy
DL1 (n=3)
284 (111-348)
N/A
Monotherapy
DL1 (n=3)
Monotherapy
DL2 (n=6)
157 (99-241)
N/A
Monotherapy
DL2 (n=6)
Treatment schedule
Median of follow-up, days (range)
Median CARVac injections, n (range)
●
Safety, n
DLTS
Patients with Grade 23 AEs
AES Grade 23 suspected to be related
to BNT211
Patients with CRS
Patients with ICANS
Deaths
Disease progression
SAE
0
3
0
0
1
0
Combination
DL1 (n=3)
38 (29-156)
2 (1-6)
Combination
DL1 (n=3)
0
3
8
1
1
2
0
1
5
11
4
0
2
0
Combination
DL2 (n=4)
93 (52-127)
4 (3-5)
Combination
DL2 (n=4)
Data cut-off: March 10, 2022.
AE, adverse event; CAR, chimeric antigen receptor; CARVac, CAR T cell-amplifying RNA vaccine; CRS, cytokine release syndrome; DL, dose level; DLT, dose-limiting toxicity;
HLH, hemophagocytic lymphohistiocytosis; ICANS, immune effector cell-associated neurotoxicity syndrome; SAE, serious AE.
Haanen J, et al. AACR Annual Meeting 2022; Oral presentation CT002.
1
4
22
3
0
0
0
Total
(n=16)
127 (2-348)
N/A
Total
(n=16)
2
15
45
8
1
Cell therapies
5
0
●
2 DLTs observed: prolonged pancytopenia after lymphodepletion (monotherapy DL2) and HLH (combination DL2, before start of CARVac)
All CRS were Grade 1 or 2; reported in 70% of patients at DL2 and manageable by administration of tocilizumab (if needed)
BIONTECH
135View entire presentation