Company Overview
Ajuo esn jeu
For per
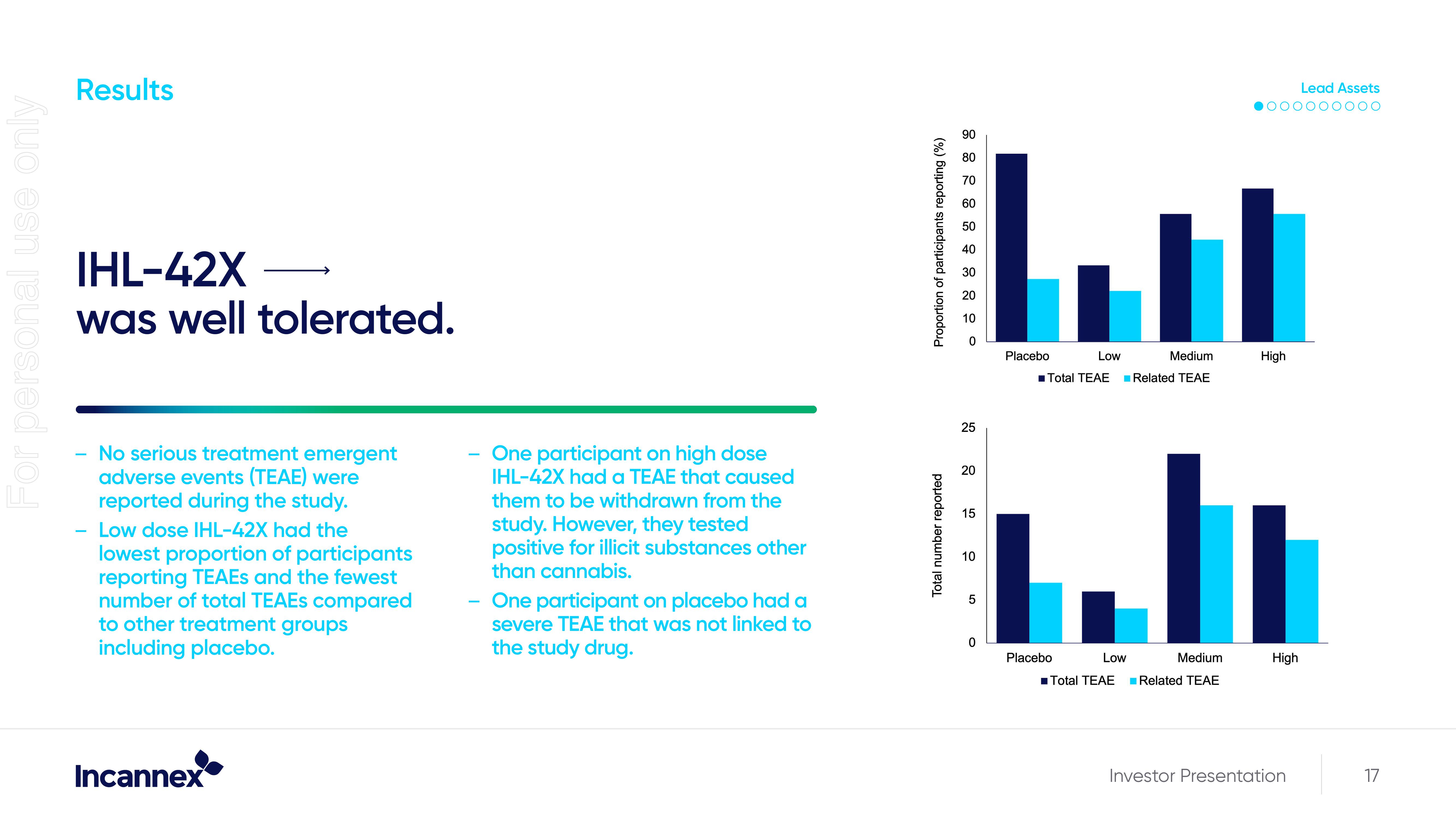
Results
IHL-42X
was well tolerated.
No serious treatment emergent
adverse events (TEAE) were
reported during the study.
- Low dose IHL-42X had the
lowest proportion of participants
reporting TEAES and the fewest
number of total TEAES compared
to other treatment groups
including placebo.
Incannex
One participant on high dose
IHL-42X had a TEAE that caused
them to be withdrawn from the
study. However, they tested
positive for illicit substances other
than cannabis.
- One participant on placebo had a
severe TEAE that was not linked to
the study drug.
Proportion of participants reporting (%)
Total number reported
90
80
70
60
50
40
30
20
10
0
25
20
15
10
5
0
Lu
Low
Placebo
■Total TEAE
Placebo
Low
■Total TEAE
Medium
Related TEAE
Lead Assets
ooooooooo
Medium
Related TEAE
High
High
Investor Presentation
17View entire presentation