Science 37 SPAC
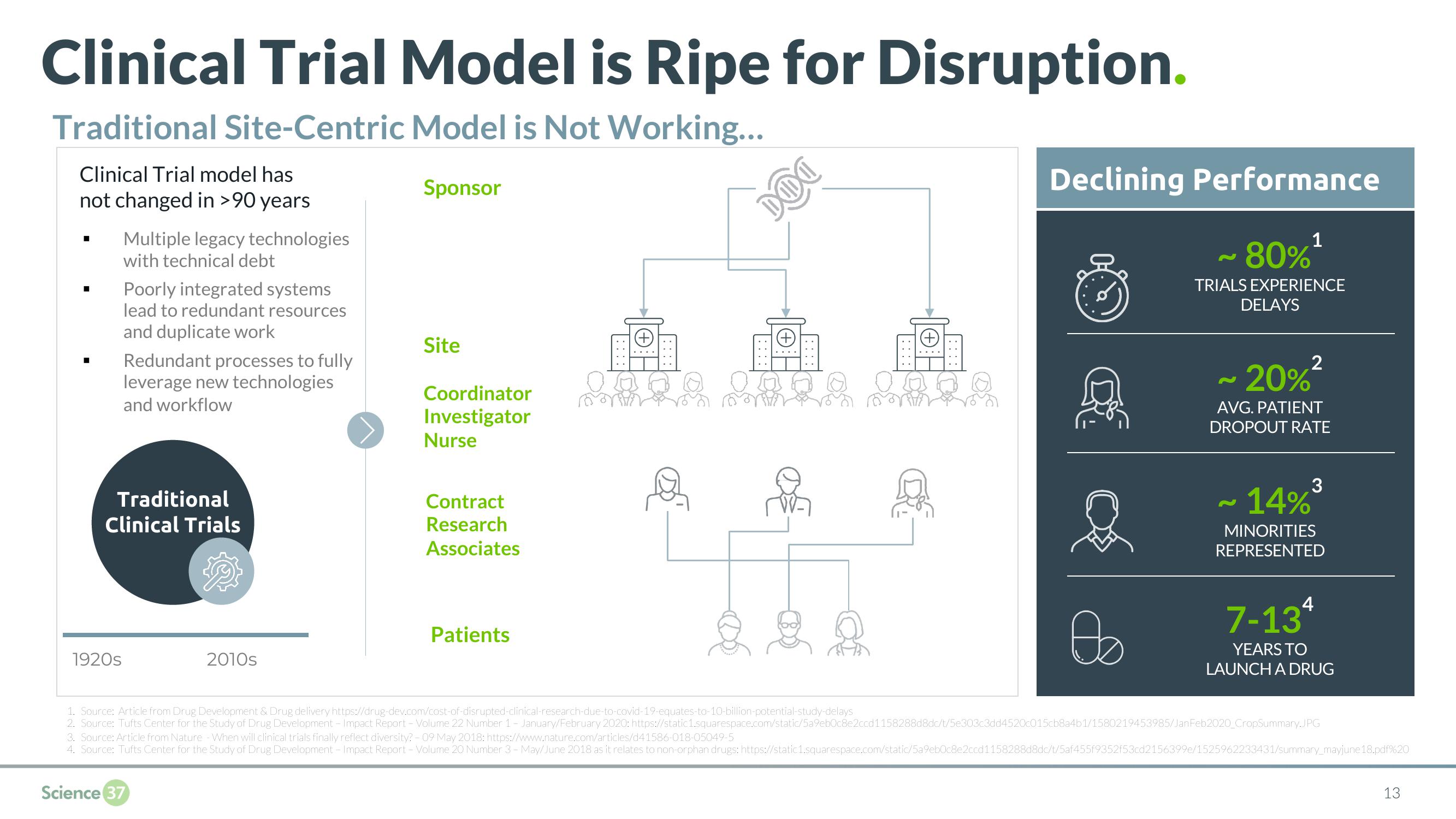
Clinical Trial Model is Ripe for Disruption.
Traditional Site-Centric Model is Not Working...
Clinical Trial model has
Sponsor
not changed in >90 years
■
■
Multiple legacy technologies
with technical debt
1920s
Poorly integrated systems
lead to redundant resources
and duplicate work
Redundant processes to fully
leverage new technologies
and workflow
Traditional
Clinical Trials
2010s
Science 37
Site
Coordinator
Investigator
Nurse
Contract
Research
Associates
Patients
DIDI
of
Declining Performance
1
80%*
TRIALS EXPERIENCE
DELAYS
N
2
20%*
AVG. PATIENT
DROPOUT RATE
3
14%*
MINORITIES
REPRESENTED
4
7-13
YEARS TO
LAUNCH A DRUG
1. Source: Article from Drug Development & Drug delivery https://drug-dev.com/cost-of-disrupted-clinical-research-due-to-covid-19-equates-to-10-billion-potential-study-delays
2. Source: Tufts Center for the Study of Drug Development - Impact Report - Volume 22 Number 1-January/February 2020: https://static1.squarespace.com/static/5a9eb0c8e2ccd1158288d8dc/t/5e303c3dd4520c015cb8a4b1/1580219453985/Jan Feb2020_CropSummary.JPG
3. Source: Article from Nature - When will clinical trials finally reflect diversity? - 09 May 2018: https://www.nature.com/articles/d41586-018-05049-5
4. Source: Tufts Center for the Study of Drug Development - Impact Report - Volume 20 Number 3-May/June 2018 as it relates to non-orphan drugs: https://static1.squarespace.com/static/5a9eb0c8e2ccd1158288d8dc/t/5af455f9352f53cd2156399e/1525962233431/summary_mayjune 18.pdf%20
13View entire presentation