BioAtla IPO Presentation Deck
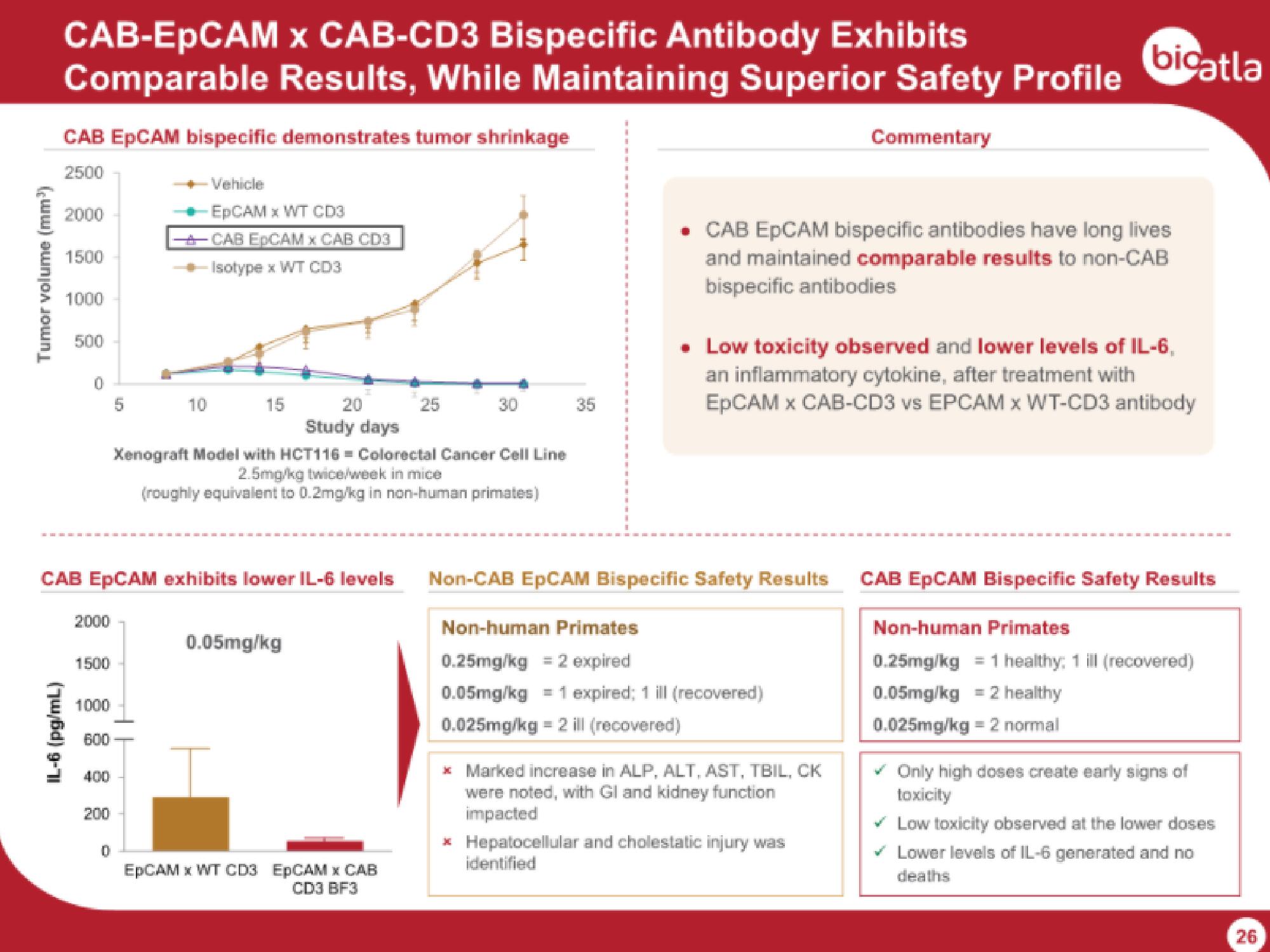
Tumor volume (mm³)
CAB-EpCAM x CAB-CD3 Bispecific Antibody Exhibits
Comparable Results, While Maintaining Superior Safety Profile bicatla
Commentary
IL-6 (pg/mL)
CAB EpCAM bispecific demonstrates tumor shrinkage
2500
2000
1500
1000
500
0
2000
1500
1000
CAB EpCAM exhibits lower IL-6 levels
600
400
200
5
0
-Vehicle
-EpCAM x WT CD3
CAB EDCAM x CAB CD3
-Isotype x WT CD3
20
Study days
Xenograft Model with HCT116= Colorectal Cancer Cell Line
2.5mg/kg twice/week in mice
(roughly equivalent to 0.2mg/kg in non-human primates)
10
15
0.05mg/kg
25
EpCAM x WT CD3 EpCAM x CAB
CD3 BF3
30
35
• CAB EpCAM bispecific antibodies have long lives
and maintained comparable results to non-CAB
bispecific antibodies
. Low toxicity observed and lower levels of IL-6,
an inflammatory cytokine, after treatment with
EpCAM x CAB-CD3 vs EPCAM x WT-CD3 antibody
Non-CAB EpCAM Bispecific Safety Results
Non-human Primates
0.25mg/kg = 2 expired
0.05mg/kg = 1 expired; 1 ill (recovered)
0.025mg/kg = 2 ill (recovered)
* Marked increase in ALP, ALT, AST, TBIL, CK
were noted, with Gl and kidney function
impacted
* Hepatocellular and cholestatic injury was
identified
CAB EpCAM Bispecific Safety Results
Non-human Primates
0.25mg/kg = 1 healthy; 1 ill (recovered)
0.05mg/kg = 2 healthy
0.025mg/kg = 2 normal
✓ Only high doses create early signs of
toxicity
Low toxicity observed at the lower doses
Lower levels of IL-6 generated and no
deaths
26View entire presentation