Equillium Results Presentation Deck
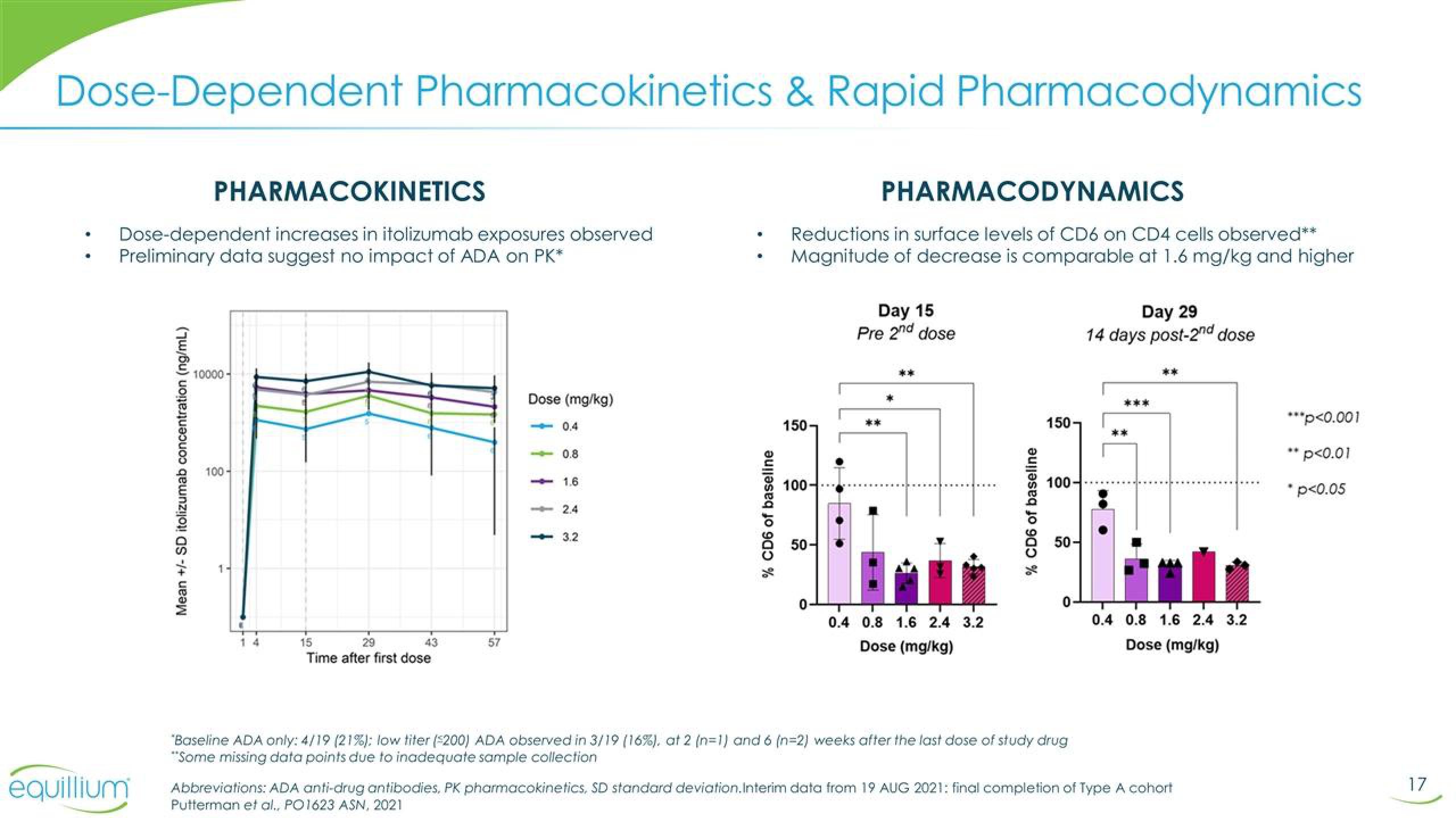
Dose-Dependent Pharmacokinetics & Rapid Pharmacodynamics
•
PHARMACOKINETICS
Dose-dependent increases in itolizumab exposures observed
Preliminary data suggest no impact of ADA on PK*
equillium
Mean +/- SD itolizumab concentration (ng/mL)
10000-
100
15
43
29
Time after first dose
57
Dose (mg/kg)
0.4
0.8
1.6
2.4
3.2
% CD6 of baseline
PHARMACODYNAMICS
Reductions in surface levels of CD6 on CD4 cells observed**
Magnitude of decrease is comparable at 1.6 mg/kg and higher
150
100-
50-
0.
0.4
Day 15
Pre 2nd dose
0.8 1.6 2.4 3.2
Dose (mg/kg)
% CD6 of baseline
150
100
50-
0
"Baseline ADA only: 4/19 (21%): low titer (200) ADA observed in 3/19 (16%), at 2 (n=1) and 6 (n=2) weeks after the last dose of study drug
"Some missing data points due to inadequate sample collection
Day 29
14 days post-2nd dose
**
in
0.4 0.8 1.6 2.4 3.2
Dose (mg/kg)
Abbreviations: ADA anti-drug antibodies, PK pharmacokinetics, SD standard deviation. Interim data from 19 AUG 2021: final completion of Type A cohort
Putterman et al., PO1623 ASN, 2021
***p<0.001
** p<0.01
*p<0.05
17View entire presentation