Kymera Results Presentation Deck
●
●
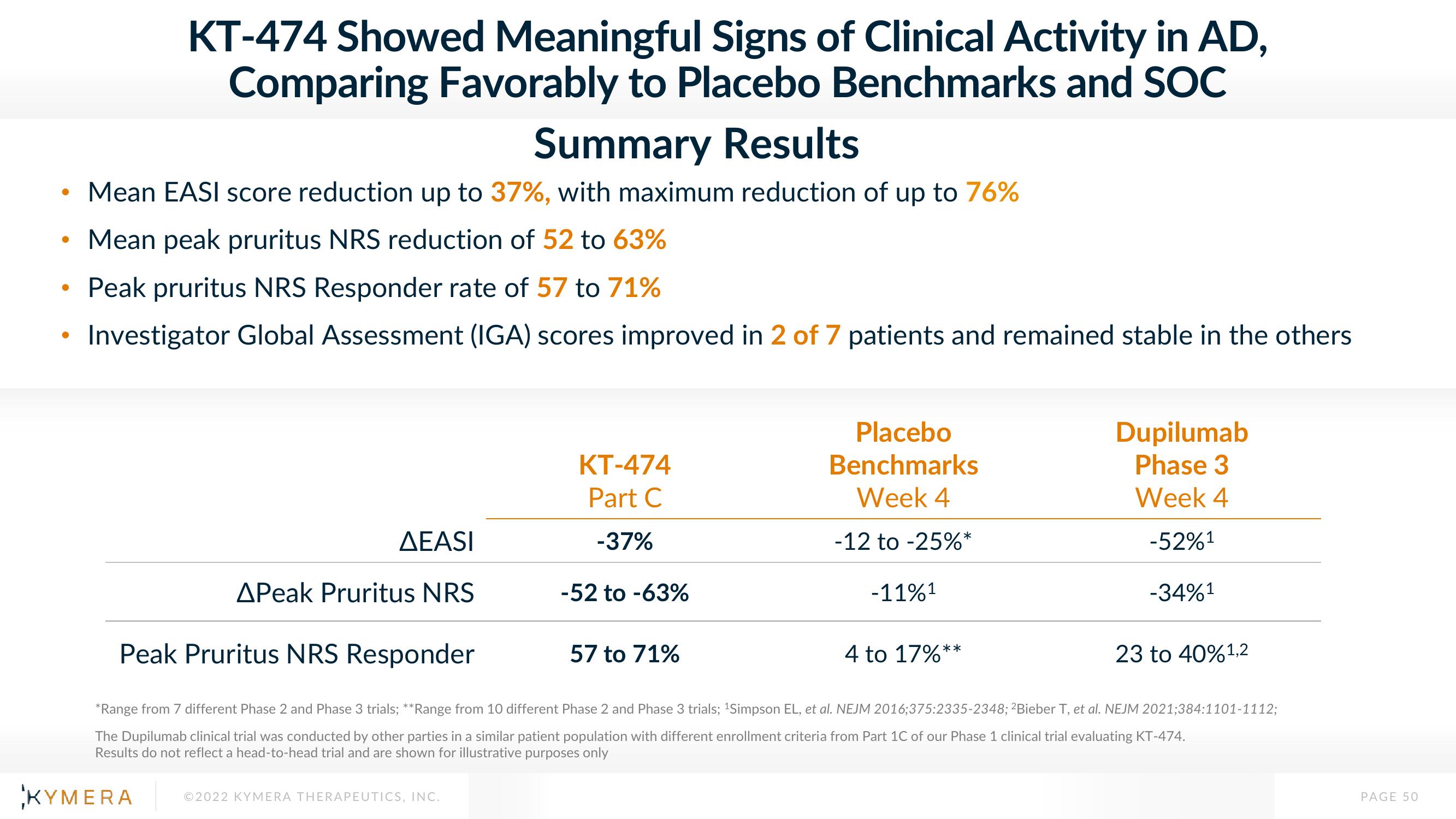
KT-474 Showed Meaningful Signs of Clinical Activity in AD,
Comparing Favorably to Placebo Benchmarks and SOC
Summary Results
Mean EASI score reduction up to 37%, with maximum reduction of up to 76%
Mean peak pruritus NRS reduction of 52 to 63%
Peak pruritus NRS Responder rate of 57 to 71%
Investigator Global Assessment (IGA) scores improved in 2 of 7 patients and remained stable in the others
AEASI
APeak Pruritus NRS
KT-474
Part C
-37%
-52 to -63%
Placebo
Benchmarks
Week 4
-12 to -25%*
57 to 71%
-11%¹
Peak Pruritus NRS Responder
*Range from 7 different Phase 2 and Phase 3 trials; **Range from 10 different Phase 2 and Phase 3 trials; ¹Simpson EL, et al. NEJM 2016;375:2335-2348; 2Bieber T, et al. NEJM 2021;384:1101-1112;
The Dupilumab clinical trial was conducted by other parties in a similar patient population with different enrollment criteria from Part 1C of our Phase 1 clinical trial evaluating KT-474.
Results do not reflect a head-to-head trial and are shown for illustrative purposes only
KYMERA Ⓒ2022 KYMERA THERAPEUTICS, INC.
**
Dupilumab
Phase 3
Week 4
-52%¹
-34%¹
4 to 17%*
23 to 40%¹,2
PAGE 50View entire presentation