Ocuphire Pharma Results
APX3330 has the Potential to be First Line of Therapy for DR Patients
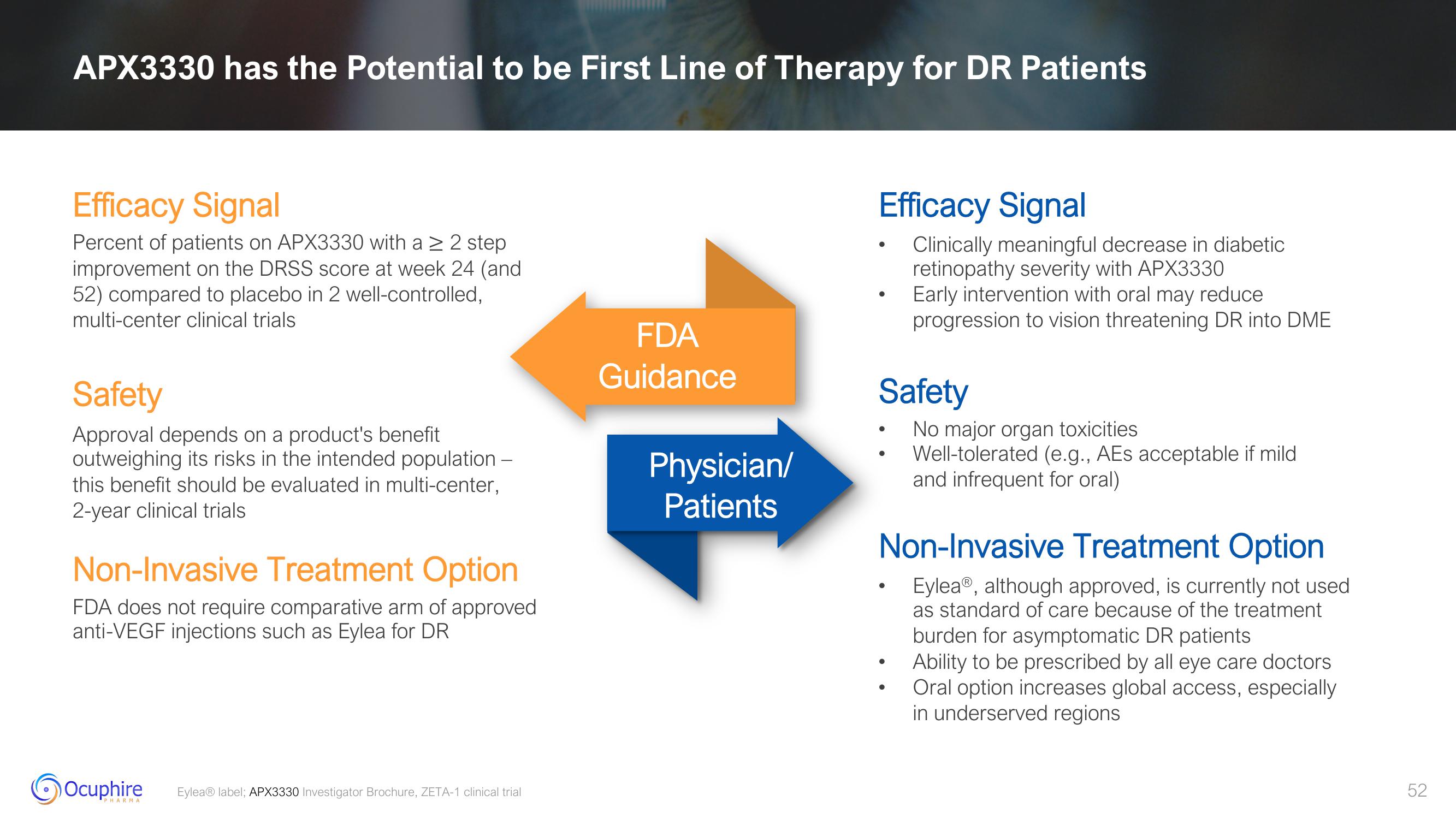
Efficacy Signal
Percent of patients on APX3330 with a ≥ 2 step
improvement on the DRSS score at week 24 (and
52) compared to placebo in 2 well-controlled,
multi-center clinical trials
Safety
Approval depends on a product's benefit
outweighing its risks in the intended population -
this benefit should be evaluated in multi-center,
2-year clinical trials
Non-Invasive Treatment Option
FDA does not require comparative arm of approved
anti-VEGF injections such as Eylea for DR
Ocuphire Eylea® label; APX3330 Investigator Brochure, ZETA-1 clinical trial
PHARMA
FDA
Guidance
Physician/
Patients
Efficacy Signal
Clinically meaningful decrease in diabetic
retinopathy severity with APX3330
●
●
Safety
●
Early intervention with oral may reduce
progression to vision threatening DR into DME
●
Non-Invasive Treatment Option
Eylea, although approved, is currently not used
as standard of care because of the treatment
burden for asymptomatic DR patients
Ability to be prescribed by all eye care doctors.
Oral option increases global access, especially
in underserved regions
●
No major organ toxicities
Well-tolerated (e.g., AEs acceptable if mild
and infrequent for oral)
52View entire presentation