AstraZeneca Results Presentation Deck
Oncology: 2021 ASCO Annual Meeting
Further solid progress in redefining cancer care
2021 ASCO Annual Meeting: another strong
presence - 90 abstracts and 74 presentations¹
One plenary session (Lynparza OlympiA Phase III
trial; adjuvant breast cancer); 12 oral presentations;
14 poster discussions; 47 posters; and 16 abstracts
(publication only)
Calquence
ELEVATE-TN Phase III four-year follow-up
ELEVATE-RR Phase III vs. ibrutinib
Imfinzi
PACIFIC Phase III five-year overall survival
Enhertu, datopotamab deruxtecan, other
potential new medicines from the pipeline
1. 24 additional presentations at ASCO 2021 featured AstraZeneca medicines and potential new medicines but were not supported by
AstraZeneca. Source: ASCO 2021 accepted abstracts.
31
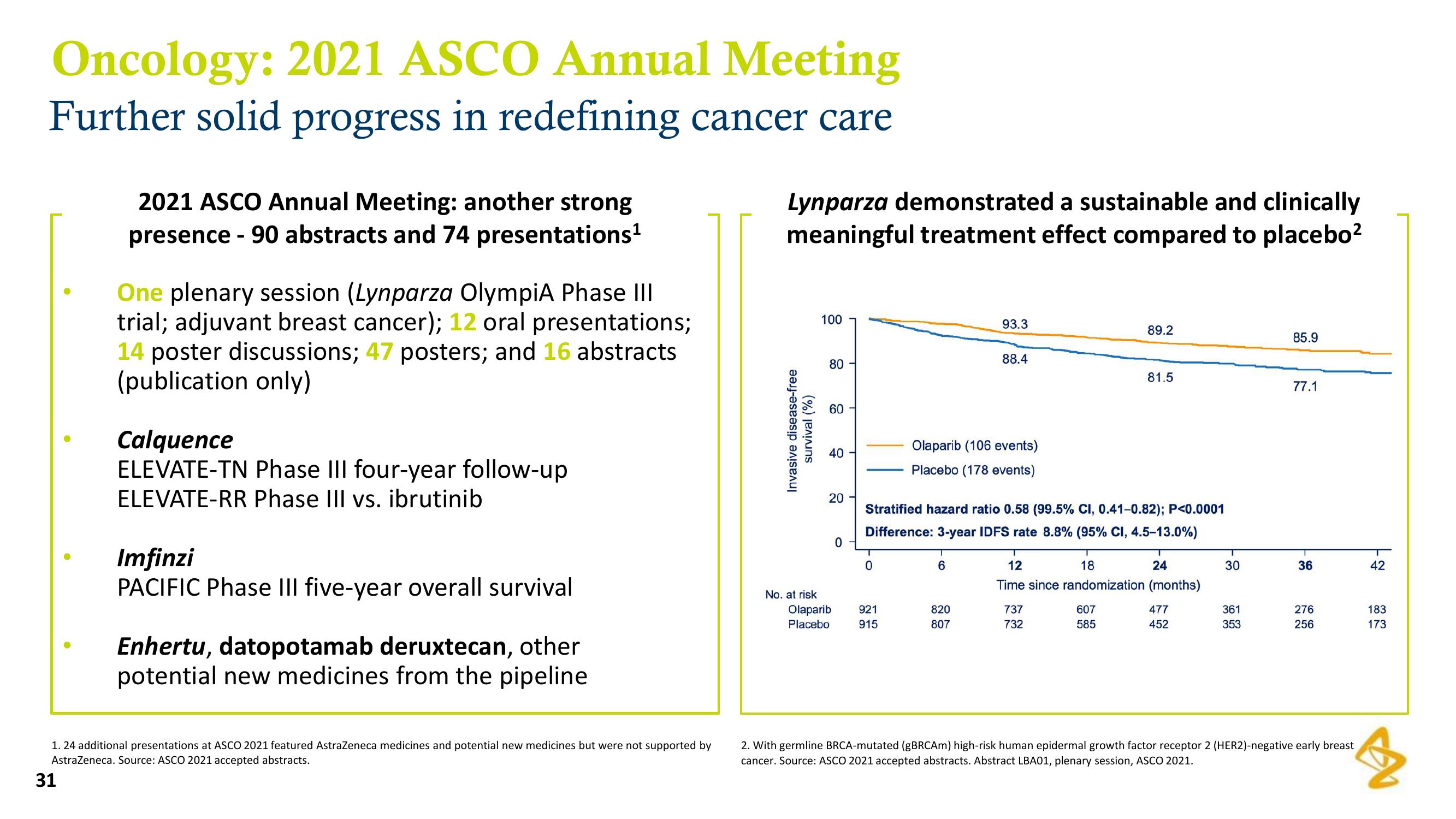
Lynparza demonstrated a sustainable and clinically
meaningful treatment effect compared to placebo²
Invasive disease-free
survival (%)
No. at risk
100
80
60
40
20
Olaparib
Placebo
0
0
921
915
93.3
Olaparib (106 events)
Placebo (178 events)
T
6
88.4
Stratified hazard ratio 0.58 (99.5% CI, 0.41-0.82); P<0.0001
Difference: 3-year IDFS rate 8.8% (95% CI, 4.5-13.0%)
T
T
24
18
820
807
89.2
737
732
81.5
T
12
Time since randomization (months)
607
585
477
452
T
30
361
353
85.9
77.1
36
276
256
42
183
173
2. With germline BRCA-mutated (gBRCAm) high-risk human epidermal growth factor receptor 2 (HER2)-negative early breast
cancer. Source: ASCO 2021 accepted abstracts. Abstract LBA01, plenary session, ASCO 2021.
3View entire presentation