BenevolentAI Investor Day Presentation Deck
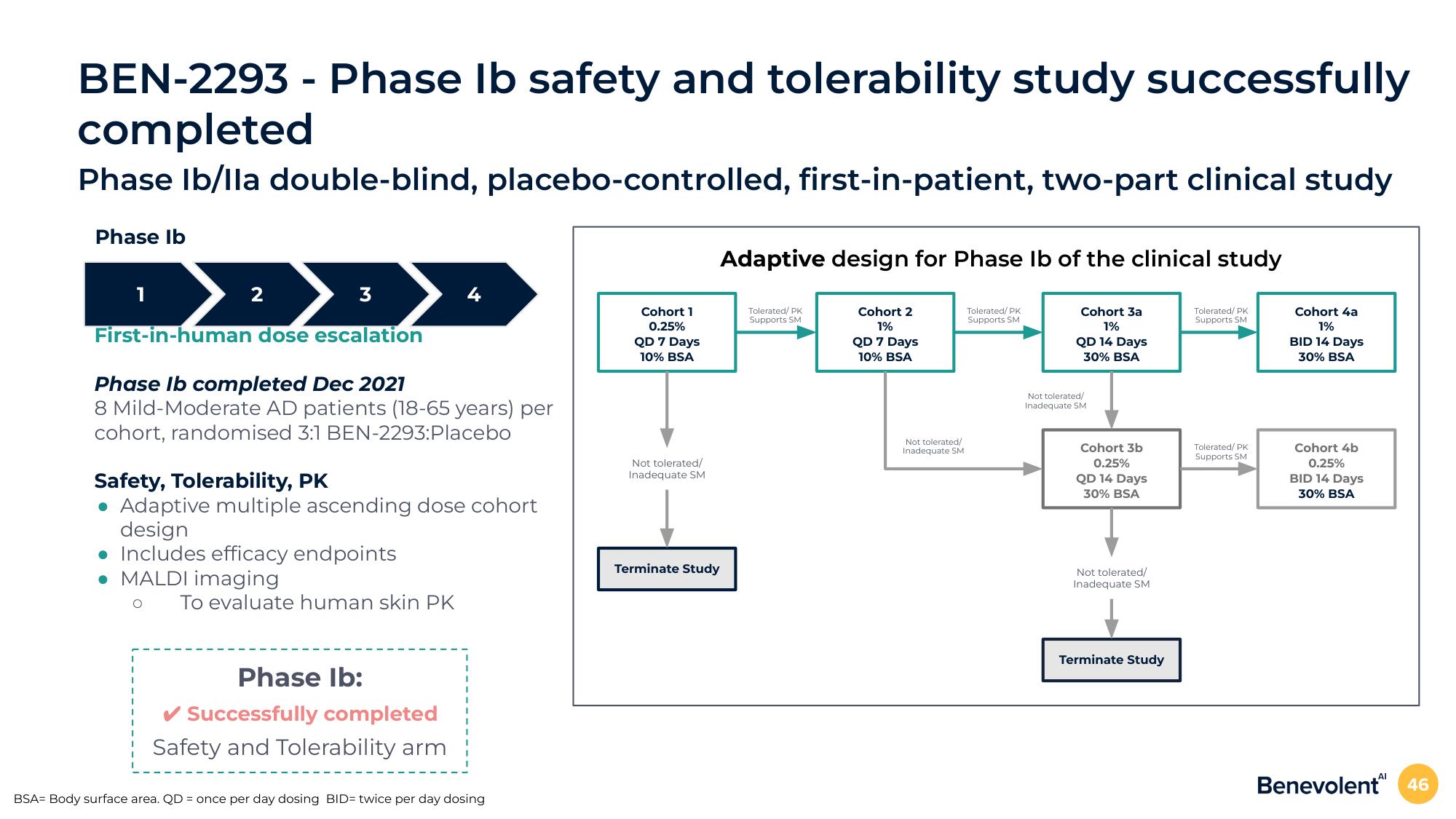
BEN-2293 - Phase Ib safety and tolerability study successfully
completed
Phase Ib/Ila double-blind, placebo-controlled, first-in-patient, two-part clinical study
Phase Ib
3
First-in-human dose escalation
2
Phase Ib completed Dec 2021
8 Mild-Moderate AD patients (18-65 years) per
cohort, randomised 3:1 BEN-2293:Placebo
Safety, Tolerability, PK
• Adaptive multiple ascending dose cohort
design
Includes efficacy endpoints
• MALDI imaging
To evaluate human skin PK
Phase Ib:
✔ Successfully completed
Safety and Tolerability arm
BSA= Body surface area. QD = once per day dosing BID= twice per day dosing
Cohort 1
0.25%
QD 7 Days
10% BSA
Not tolerated/
Inadequate SM
Adaptive design for Phase Ib of the clinical study
Cohort 3a
1%
Cohort 2
1%
QD 7 Days
10% BSA
Terminate Study
Tolerated/PK
Supports SM
Not tolerated/
Inadequate SM
Tolerated/PK
Supports SM
QD 14 Days
30% BSA
Not tolerated/
Inadequate SM
Cohort 3b
0.25%
QD 14 Days
30% BSA
Not tolerated/
Inadequate SM
Terminate Study
Tolerated/PK
Supports SM
Tolerated/PK
Supports SM
Cohort 4a
1%
BID 14 Days
30% BSA
Cohort 4b
0.25%
BID 14 Days
30% BSA
Benevolent 46View entire presentation