Restore Mind Medicine
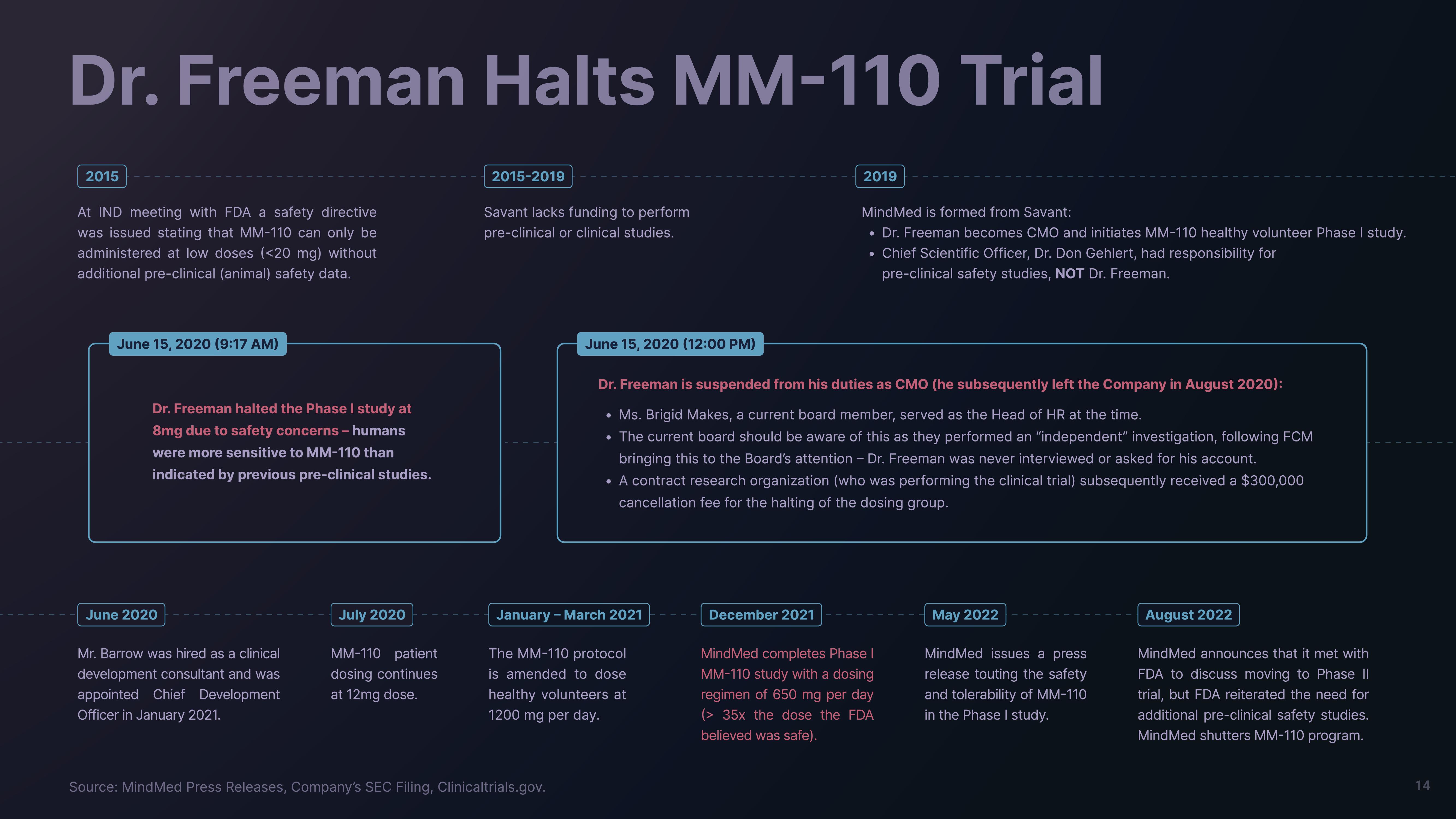
Dr. Freeman Halts MM-110 Trial
2015
At IND meeting with FDA a safety directive
was issued stating that MM-110 can only be
administered at low doses (<20 mg) without
additional pre-clinical (animal) safety data.
June 15, 2020 (9:17 AM)
Dr. Freeman halted the Phase I study at
8mg due to safety concerns-humans
were more sensitive to MM-110 than
indicated by previous pre-clinical studies.
June 2020
Mr. Barrow was hired as a clinical
development consultant and was
appointed Chief Development
Officer in January 2021.
July 2020
MM-110 patient
dosing continues
at 12mg dose.
2015-2019
Savant lacks funding to perform
pre-clinical or clinical studies.
January - March 2021
The MM-110 protocol
is amended to dose
healthy volunteers at
1200 mg per day.
Source: MindMed Press Releases, Company's SEC Filing, Clinicaltrials.gov.
2019
June 15, 2020 (12:00 PM)
Dr. Freeman is suspended from his duties as CMO (he subsequently left the Company in August 2020):
• Ms. Brigid Makes, a current board member, served as the Head of HR at the time.
The current board should be aware of this as they performed an "independent" investigation, following FCM
bringing this to the Board's attention - Dr. Freeman was never interviewed or asked for his account.
• A contract research organization (who was performing the clinical trial) subsequently received a $300,000
cancellation fee for the halting of the dosing group.
December 2021
MindMed is formed from Savant:
• Dr. Freeman becomes CMO and initiates MM-110 healthy volunteer Phase I study.
• Chief Scientific Officer, Dr. Don Gehlert, had responsibility for
pre-clinical safety studies, NOT Dr. Freeman.
MindMed completes Phase I
MM-110 study with a dosing
regimen of 650 mg per day
(> 35x the dose the FDA
believed was safe).
May 2022
MindMed issues a press
release touting the safety
and tolerability of MM-110
in the Phase I study.
August 2022
MindMed announces that it met with
FDA to discuss moving to Phase II
trial, but FDA reiterated the need for
additional pre-clinical safety studies.
MindMed shutters MM-110 program.
14View entire presentation