Investor Presentation
Biologic Activity of Lenabasum in Patients
with Targeted Diseases.
16 X
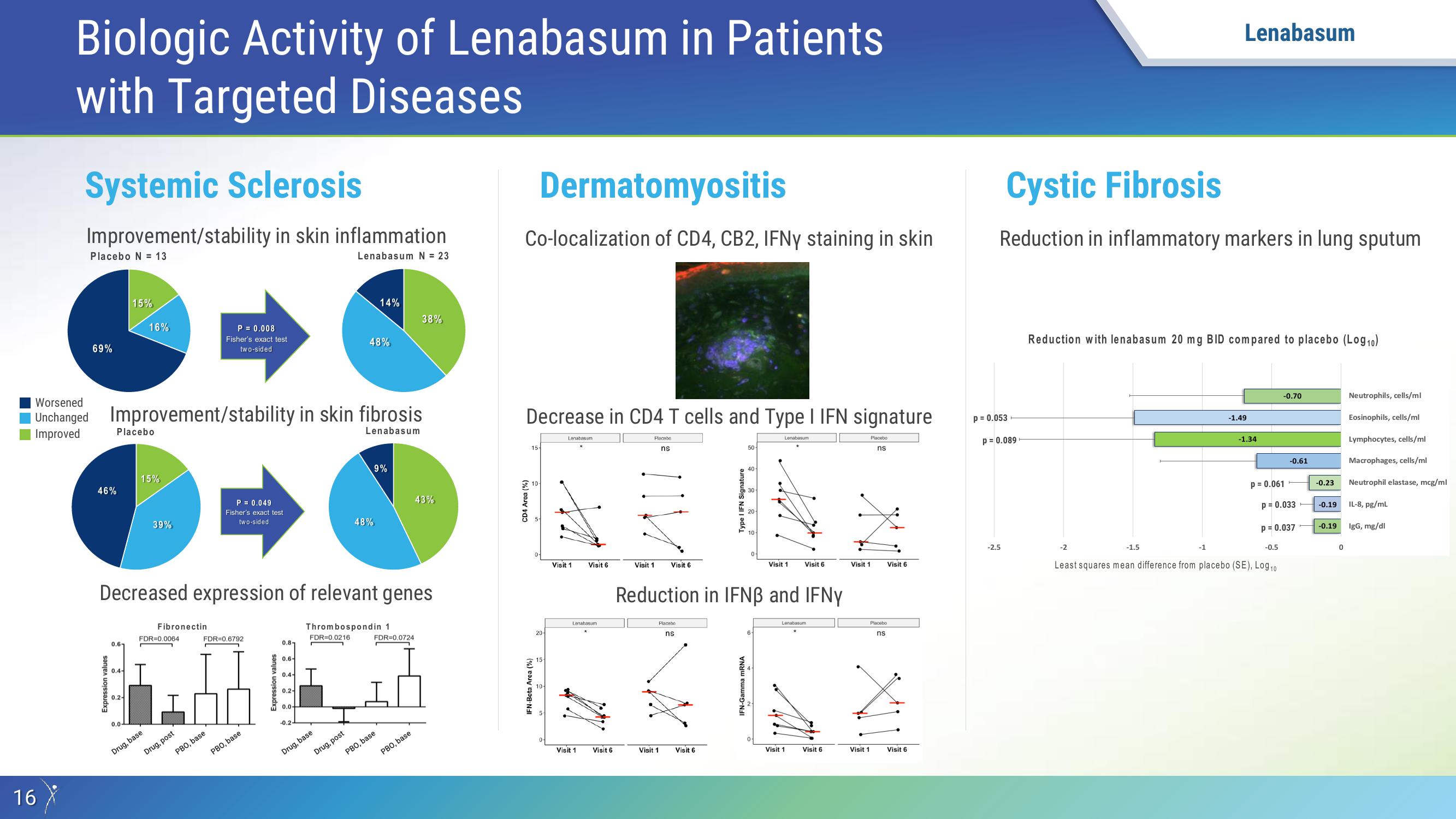
Systemic Sclerosis
Improvement/stability in skin inflammation
Placebo N = 13
Lenabasum N = 23
69%
46%
Placebo
0.6
15%
Worsened
Unchanged Improvement/stability in skin fibrosis.
Improved
Lenabasum
0.4-
0.2-
16%
0.0
15%
39%
Drug, base
Fibronectin
FDR=0.0064
P = 0.008
Fisher's exact test
two-sided
Decreased expression of relevant genes
Drug, post
PBO, base
P = 0.049
Fisher's exact test
two-sided
FDR=0.6792
PBO, base
Expression values
0.8-
0.6-
0.4-
0.2-
0.0
-0.2
48%
Drug, base
14%
Drug, post
9%
48%
Thrombospondin 1
FDR=0.0216
PBO, base
FDR=0.0724
38%
PBO, base
43%
Dermatomyositis
Co-localization of CD4, CB2, IFNy staining in skin
Decrease in CD4 T cells and Type I IFN signature
CD4 Area (%)
15
FN-Beta Area (%)
20
Lenabasum
*
Visit 1
Visit 6
Lenabasum
Visit 1
Visit 6
Placebo
ns
1]]
Visit 1
Visit 6
Placebo
Visit 1
ns
Type I IFN Signature
Visit 6
50
IFN-Gamma mRNA
40
Reduction in IFNB and IFNY
Lenabasum
MA
Visit 1
Visit 6
Lenabasum
Visit 1
Visit 6
Visit 1
Visit 1
Placebo
ns
Visit 6
Placebo
ns
Visit 6
Cystic Fibrosis
Reduction in inflammatory markers in lung sputum
p = 0.053
p = 0.089
-2.5
Reduction with lenabasum 20 mg BID compared to placebo (Log10)
-2
Lenabasum
-1.5
-1
-1.49
-1.34
-0.70
p = 0.061
P = 0.033
-0.5
-0.61
p = 0.037
Least squares mean difference from placebo (SE), Log 10
-0.23
-0.19
-0.19
0
Neutrophils, cells/ml
Eosinophils, cells/ml
Lymphocytes, cells/ml
Macrophages, cells/ml
Neutrophil elastase, mcg/ml
IL-8, pg/mL
IgG, mg/dlView entire presentation