Immix Biopharma Investor Presentation Deck
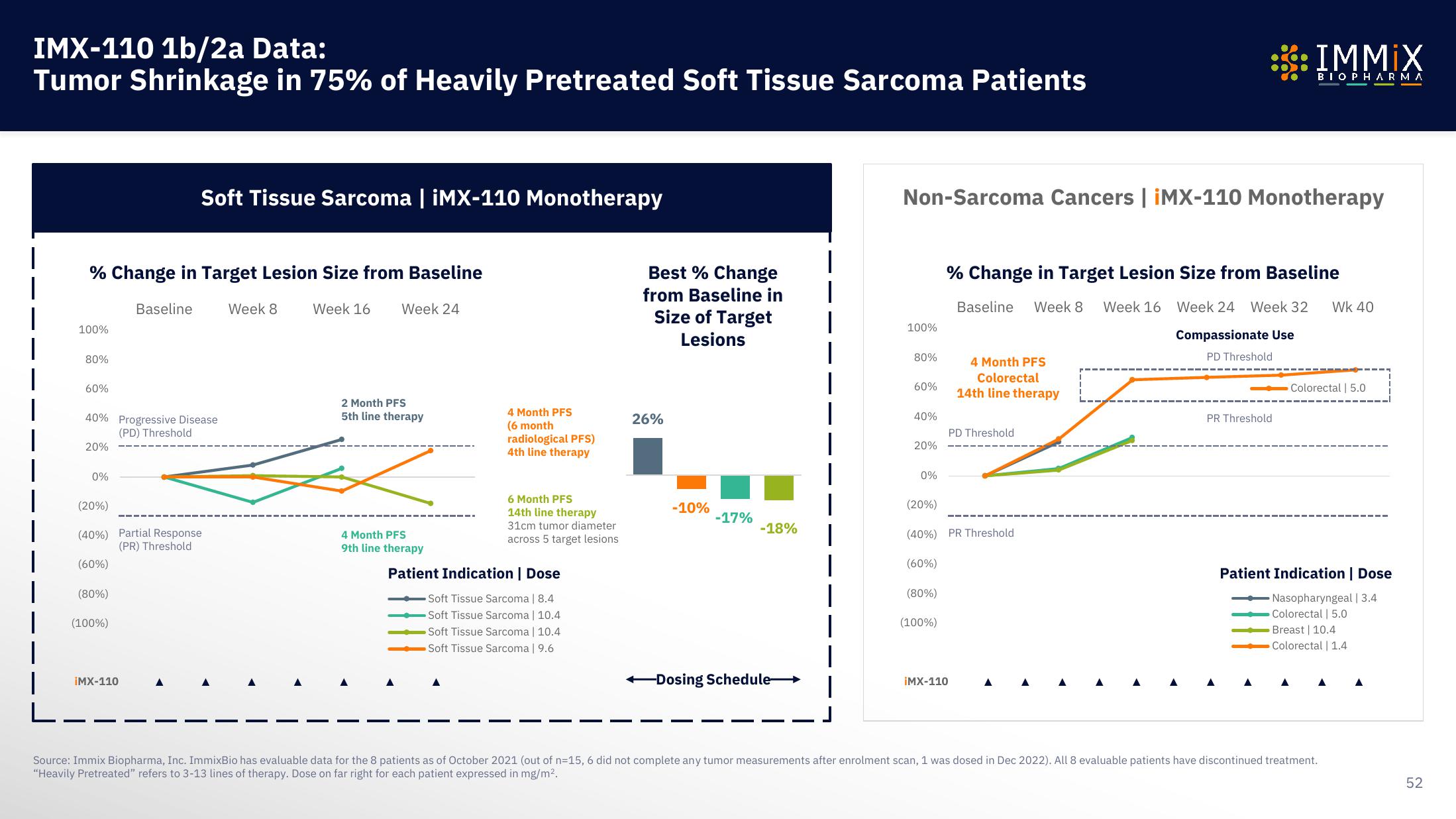
IMX-110 1b/2a Data:
Tumor Shrinkage in 75% of Heavily Pretreated Soft Tissue Sarcoma Patients
|
|
I
I
% Change in Target Lesion Size from Baseline
Week 16 Week 24
100%
80%
60%
20%
40% Progressive Disease
(PD) Threshold
0%
Soft Tissue Sarcoma | iMX-110 Monotherapy
Baseline
¡MX-110
(20%)
I
(40%) Partial Response
(PR) Threshold
|
(60%)
I
(80%)
| (100%)
I
Week 8
2 Month PFS
5th line therapy
4 Month PFS
9th line therapy
4 Month PFS
(6 month
radiological PFS)
4th line therapy
6 Month PFS
14th line therapy
31cm tumor diameter
across 5 target lesions
Patient Indication | Dose
Soft Tissue Sarcoma | 8.4
-Soft Tissue Sarcoma | 10.4
Soft Tissue Sarcoma | 10.4
Soft Tissue Sarcoma | 9.6
Best % Change
from Baseline in
Size of Target
Lesions
26%
-10%
-17%
-18%
Dosing Schedule-
|
100%
Non-Sarcoma Cancers | iMX-110 Monotherapy
80%
60%
40%
20%
0%
(20%)
% Change in Target Lesion Size from Baseline
Baseline Week 8 Week 16
Week 24 Week 32
Compassionate Use
PD Threshold
4 Month PFS
Colorectal
14th line therapy
iMX-110
PD Threshold
(40%) PR Threshold
(60%)
(80%)
(100%)
●●●
S
E
IMMIX
BIOPHARMA
PR Threshold
- Colorectal | 5.0
Wk 40
Patient Indication | Dose
Nasopharyngeal | 3.4
Colorectal | 5.0
Breast 10.4
Colorectal | 1.4
Source: Immix Biopharma, Inc. ImmixBio has evaluable data for the 8 patients as of October 2021 (out of n=15, 6 did not complete any tumor measurements after enrolment scan, 1 was dosed in Dec 2022). All 8 evaluable patients have discontinued treatment.
"Heavily Pretreated" refers to 3-13 lines of therapy. Dose on far right for each patient expressed in mg/m².
52View entire presentation