Imara M&A
Tumor Volume (mm³)
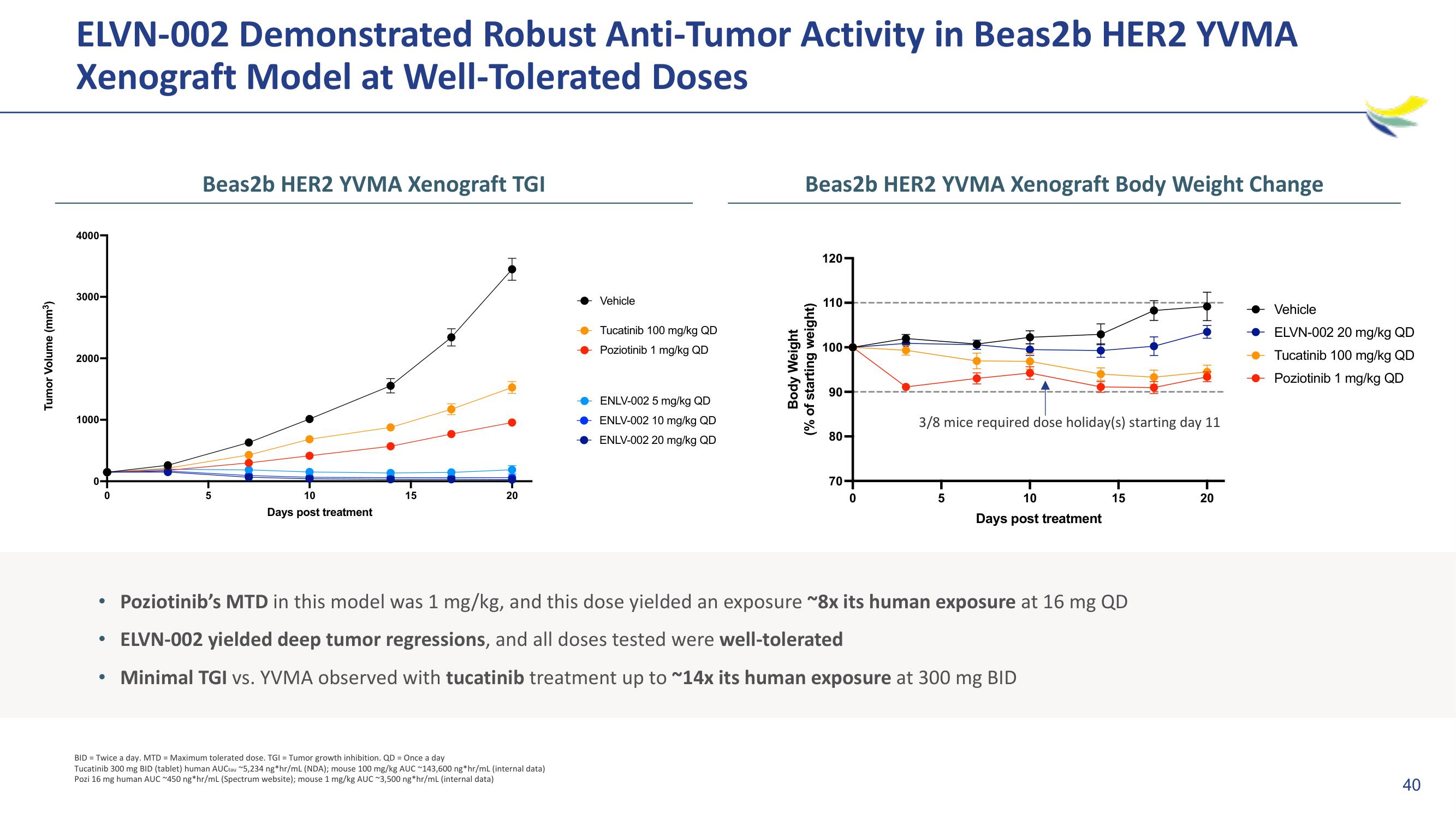
ELVN-002 Demonstrated Robust Anti-Tumor Activity in Beas2b HER2 YVMA
Xenograft Model at Well-Tolerated Doses
4000-
3000-
2000-
1000-
0
●
●
Beas2b HER2 YVMA Xenograft TGI
5
10
Days post treatment
15
20
Vehicle
BID = Twice a day. MTD = Maximum tolerated dose. TGI = Tumor growth inhibition. QD = Once a day
Tucatinib 300 mg BID (tablet) human AUCtau ~5,234 ng*hr/mL (NDA); mouse 100 mg/kg AUC ~143,600 ng*hr/mL (internal data)
Pozi 16 mg human AUC ~450 ng*hr/mL (Spectrum website); mouse 1 mg/kg AUC ~3,500 ng*hr/mL (internal data)
Tucatinib 100 mg/kg QD
Poziotinib 1 mg/kg QD
ENLV-002 5 mg/kg QD
ENLV-002 10 mg/kg QD
ENLV-002 20 mg/kg QD
Beas2b HER2 YVMA Xenograft Body Weight Change
Body Weight
(% of starting weight)
120
110-
100-
90
80-
70-
0
JOK
3/8 mice required dose holiday(s) starting day 11
5
10
Days post treatment
15
Poziotinib's MTD in this model was 1 mg/kg, and this dose yielded an exposure ~8x its human exposure at 16 mg QD
ELVN-002 yielded deep tumor regressions, and all doses tested were well-tolerated
Minimal TGI vs. YVMA observed with tucatinib treatment up to ~14x its human exposure at 300 mg BID
20
Vehicle
ELVN-002 20 mg/kg QD
Tucatinib 100 mg/kg QD
Poziotinib 1 mg/kg QD
40View entire presentation