Kymera Results Presentation Deck
●
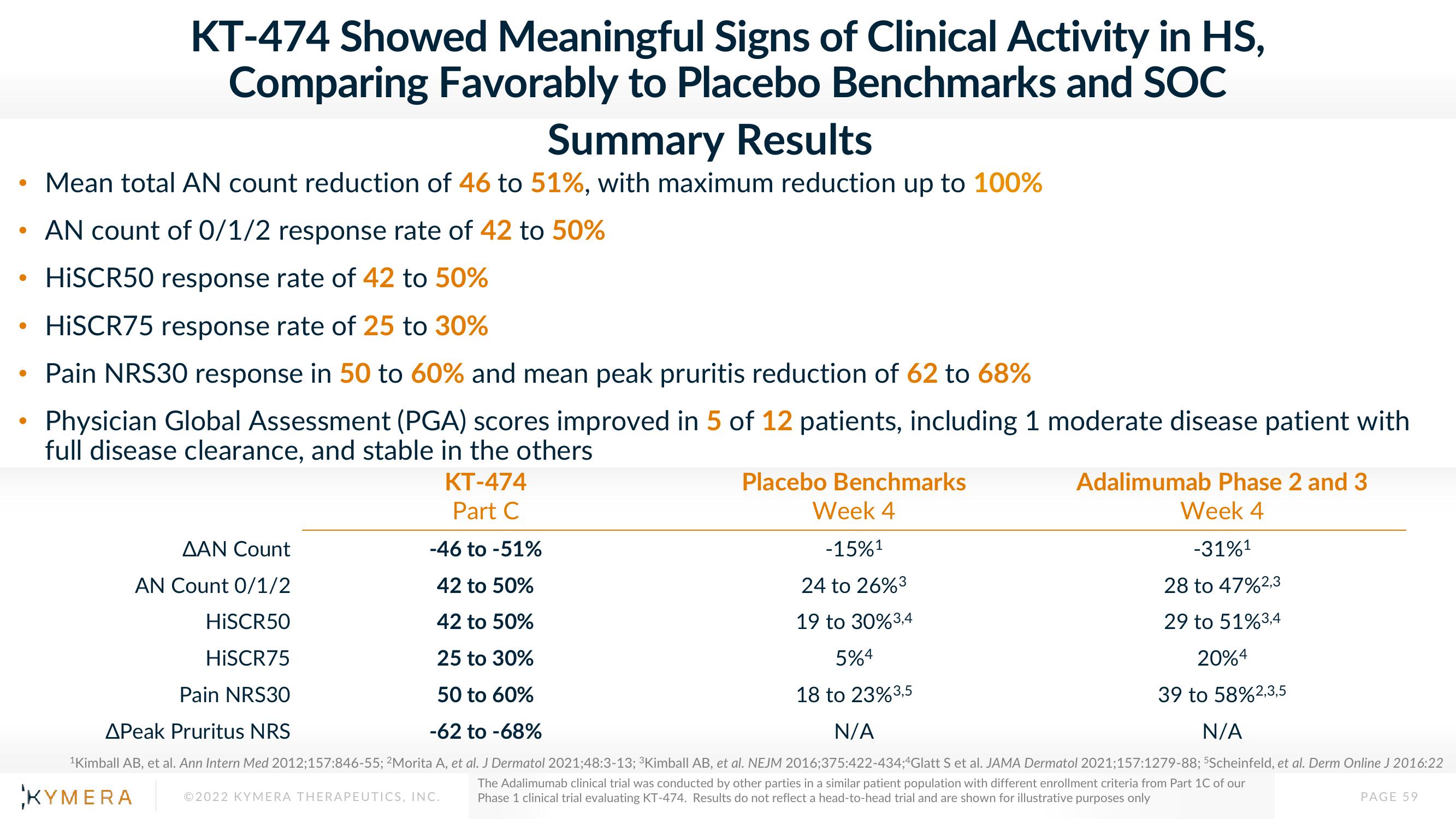
AN count of 0/1/2 response rate of 42 to 50%
HISCR50 response rate of 42 to 50%
HISCR75 response rate of 25 to 30%
Pain NRS30 response in 50 to 60% and mean peak pruritis reduction of 62 to 68%
Physician Global Assessment (PGA) scores improved in 5 of 12 patients, including 1 moderate disease patient with
full disease clearance, and stable in the others
KT-474
Part C
Placebo Benchmarks
Week 4
-15%¹
ΔΑΝ Count
-46 to -51%
AN Count 0/1/2
42 to 50%
24 to 26%3
28 to 47%2,3
HISCR50
42 to 50%
19 to 30% 3,4
5%4
29 to 51% 3,4
20%4
HISCR75
25 to 30%
Pain NRS30
50 to 60%
18 to 23% 3,5
39 to 58%2,3,5
APeak Pruritus NRS
-62 to -68%
N/A
N/A
¹Kimball AB, et al. Ann Intern Med 2012;157:846-55; 2Morita A, et al. J Dermatol 2021;48:3-13; ³Kimball AB, et al. NEJM 2016;375:422-434;4Glatt S et al. JAMA Dermatol 2021;157:1279-88; 5Scheinfeld, et al. Derm Online J 2016:22
The Adalimumab clinical trial was conducted by other parties in a similar patient population with different enrollment criteria from Part 1C of our
KYMERA
Phase 1 clinical trial evaluating KT-474. Results do not reflect a head-to-head trial and are shown for illustrative purposes only
●
●
●
KT-474 Showed Meaningful Signs of Clinical Activity in HS,
Comparing Favorably to Placebo Benchmarks and SOC
Summary Results
●
Mean total AN count reduction of 46 to 51%, with maximum reduction up to 100%
©2022 KYMERA THERAPEUTICS, INC.
Adalimumab Phase 2 and 3
Week 4
-31%¹
PAGE 59View entire presentation