Centessa IPO Presentation Deck
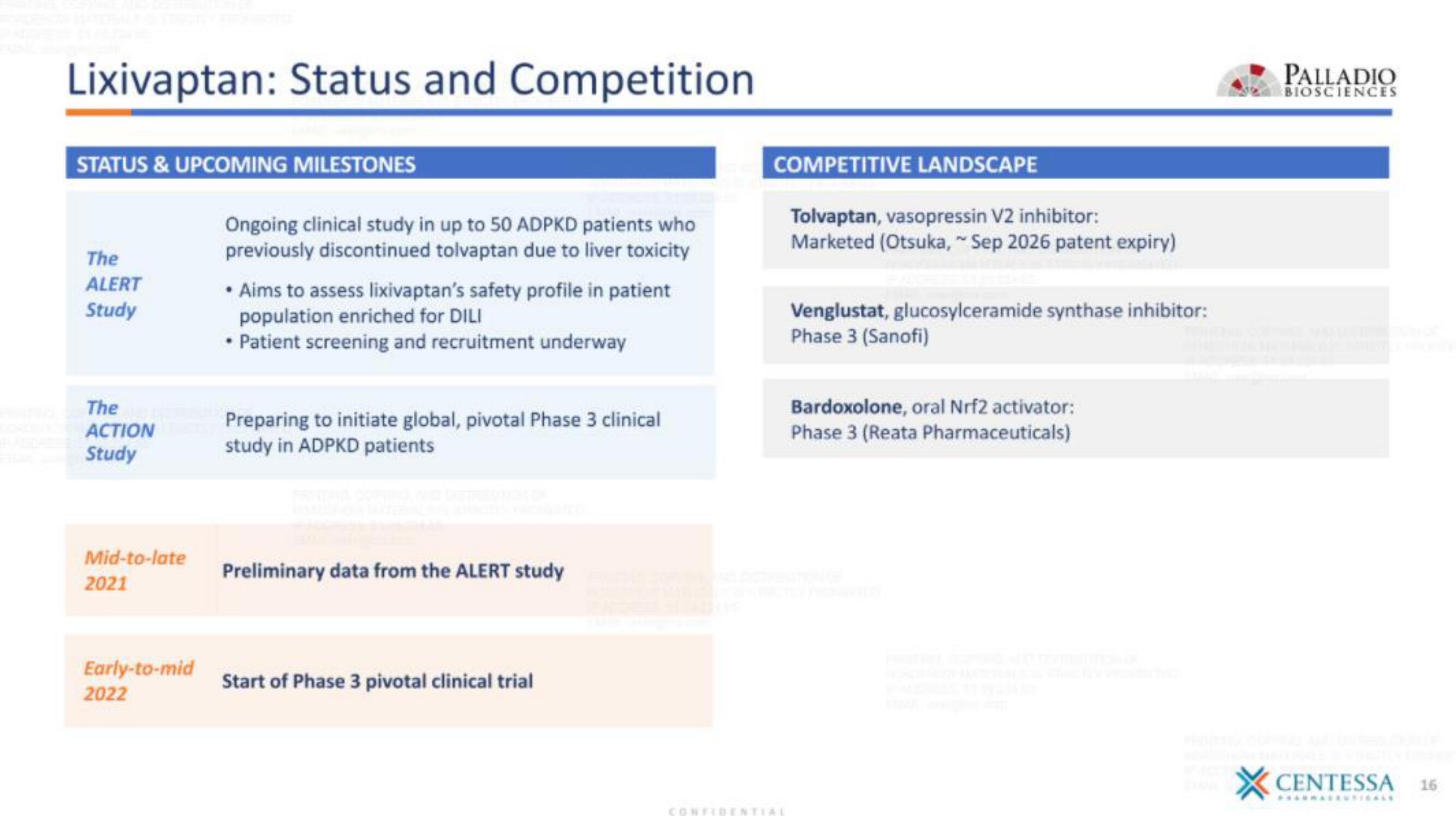
Lixivaptan: Status and Competition
STATUS & UPCOMING MILESTONES
The
ALERT
Study
The
ACTION
Study
Mid-to-late
2021
Early-to-mid
2022
Ongoing clinical study in up to 50 ADPKD patients who
previously discontinued tolvaptan due to liver toxicity
• Aims to assess lixivaptan's safety profile in patient
population enriched for DILI
• Patient screening and recruitment underway
Preparing to initiate global, pivotal Phase 3 clinical
study in ADPKD patients
Preliminary data from the ALERT study
Start of Phase 3 pivotal clinical trial
COMPETITIVE LANDSCAPE
Tolvaptan, vasopressin V2 inhibitor:
Marketed (Otsuka,~Sep 2026 patent expiry)
Venglustat, glucosylceramide synthase inhibitor:
Phase 3 (Sanofi)
Bardoxolone, oral Nrf2 activator:
Phase 3 (Reata Pharmaceuticals)
PALLADIO
BIOSCIENCES
CENTESSA 16View entire presentation