Ocuphire Pharma Results Presentation Deck
28
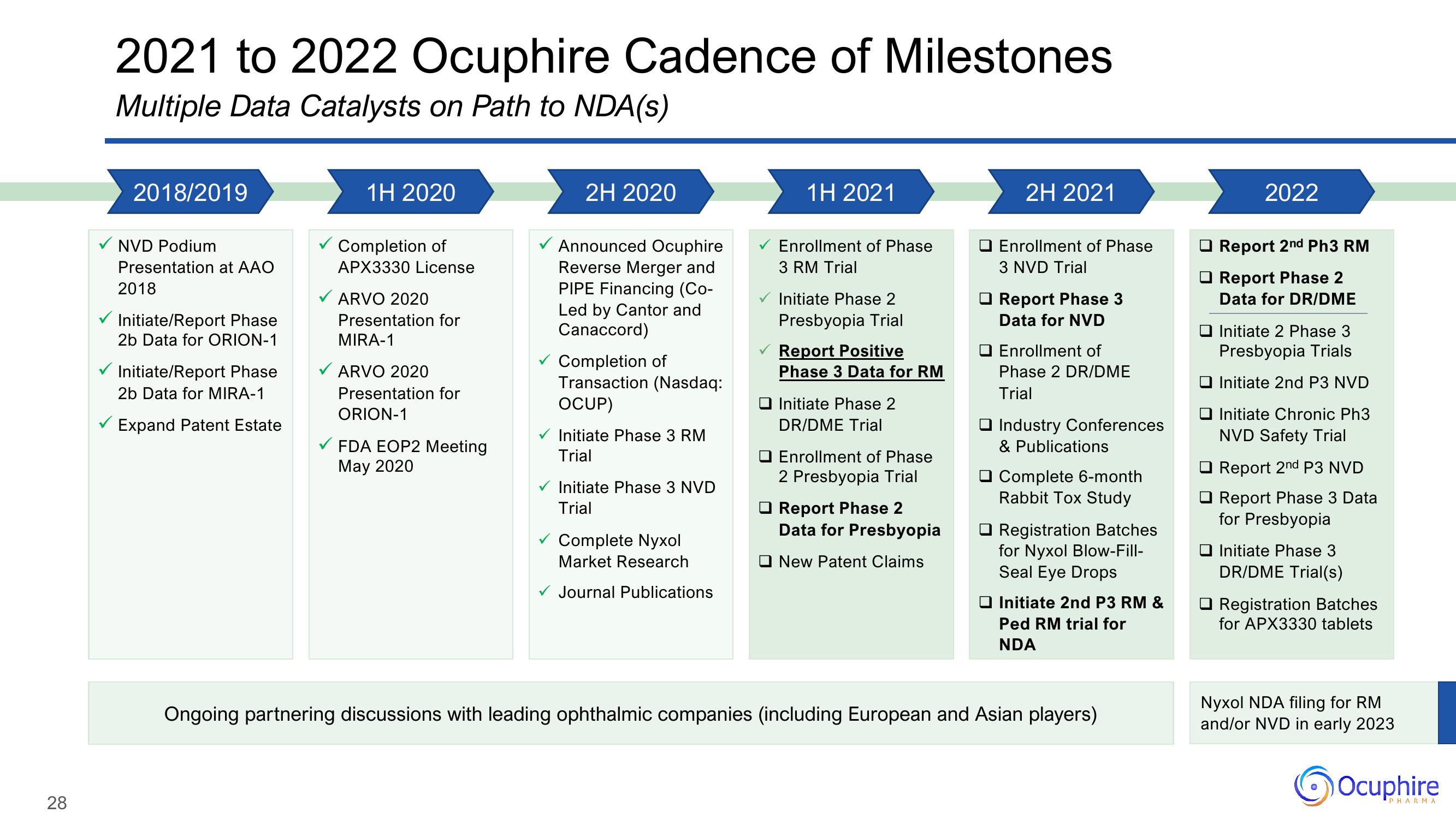
2021 to 2022 Ocuphire Cadence of Milestones
Multiple Data Catalysts on Path to NDA(s)
2018/2019
✓ NVD Podium
Presentation at AAO
2018
Initiate/Report Phase
2b Data for ORION-1
Initiate/Report Phase
2b Data for MIRA-1
Expand Patent Estate
1H 2020
Completion of
APX3330 License
ARVO 2020
Presentation for
MIRA-1
ARVO 2020
Presentation for
ORION-1
FDA EOP2 Meeting
May 2020
2H 2020
Announced Ocuphire
Reverse Merger and
PIPE Financing (Co-
Led by Cantor and
Canaccord)
✓ Completion of
Transaction (Nasdaq:
OCUP)
Initiate Phase 3 RM
Trial
✓ Initiate Phase 3 NVD
Trial
✓ Complete Nyxol
Market Research
✓ Journal Publications
1H 2021
Enrollment of Phase
3 RM Trial
Initiate Phase 2
Presbyopia Trial
Report Positive
Phase 3 Data for RM
Initiate Phase 2
DR/DME Trial
Enrollment of Phase
2 Presbyopia Trial
Report Phase 2
Data for Presbyopia
New Patent Claims
2H 2021
O Enrollment of Phase
3 NVD Trial
Report Phase 3
Data for NVD
O Enrollment of
Phase 2 DR/DME
Trial
Industry Conferences
& Publications
Complete 6-month
Rabbit Tox Study
Registration Batches
for Nyxol Blow-Fill-
Seal Eye Drops
Initiate 2nd P3 RM &
Ped RM trial for
NDA
Ongoing partnering discussions with leading ophthalmic companies (including European and Asian players)
2022
Report 2nd Ph3 RM
Report Phase 2
Data for DR/DME
Initiate 2 Phase 3
Presbyopia Trials
Initiate 2nd P3 NVD
Initiate Chronic Ph3
NVD Safety Trial
Report 2nd P3 NVD
Report Phase 3 Data
for Presbyopia
Initiate Phase 3
DR/DME Trial(s)
Registration Batches
for APX3330 tablets
Nyxol NDA filing for RM
and/or NVD in early 2023
Ocuphire
PHARMAView entire presentation