Ocuphire Pharma Investor Day Presentation Deck
April 2021
Alcon
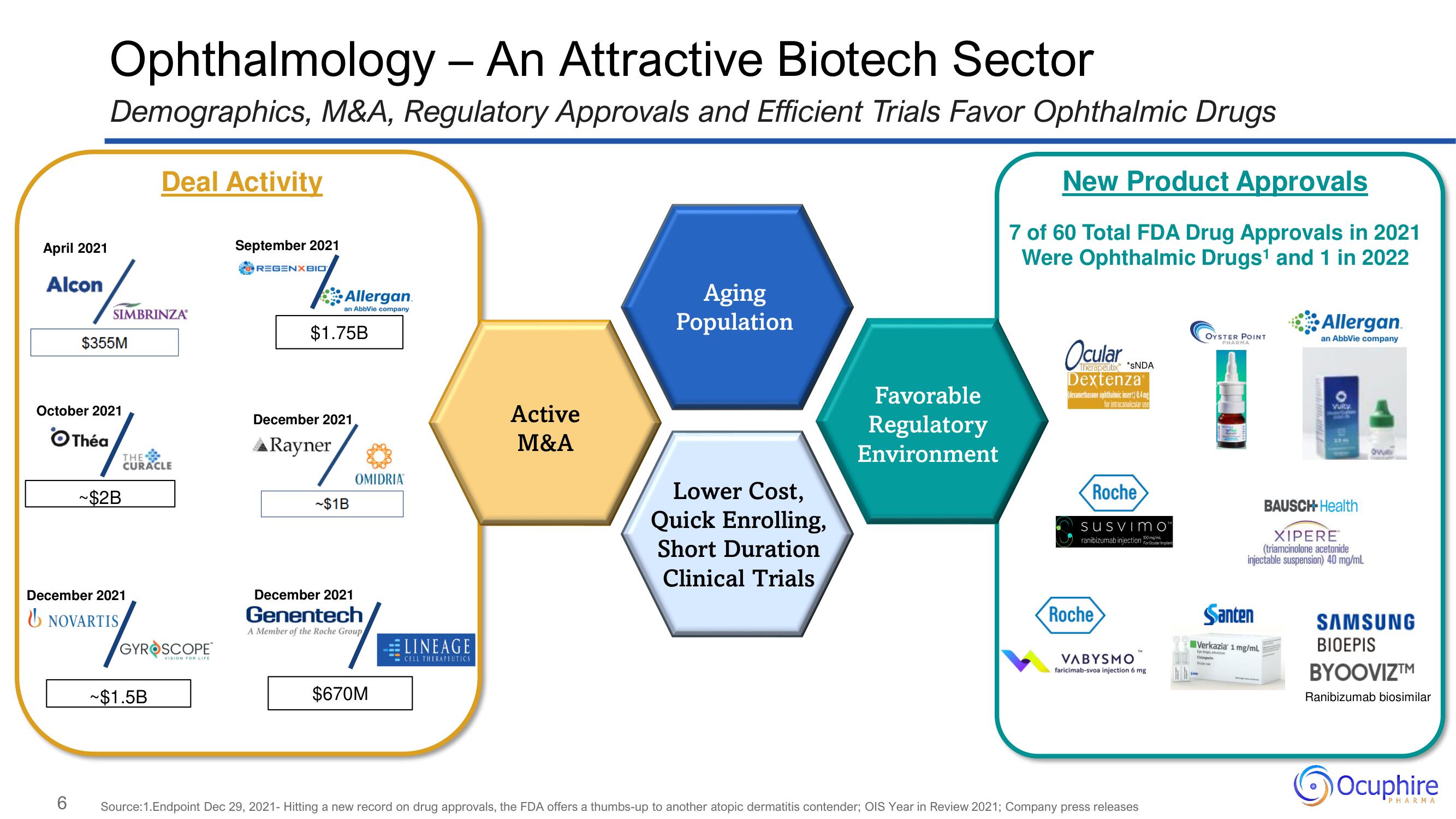
Ophthalmology - An Attractive Biotech Sector
Demographics, M&A, Regulatory Approvals and Efficient Trials Favor Ophthalmic Drugs
Deal Activity
SIMBRINZA
$355M
October 2021
Théa
6
THE
CURACLE
~$2B
December 2021
NOVARTIS
GYROSCOPE
VISION FOR LIFE
~$1.5B
September 2021
REGENXBIO
Allergan.
an AbbVie company
$1.75B
December 2021
▲Rayner
~$1B
OMIDRIA
December 2021
Genentech
A Member of the Roche Group
$670M
LINEAGE
CELL THERAPEUTICS
Active
M&A
Aging
Population
Lower Cost,
Quick Enrolling,
Short Duration
Clinical Trials
Favorable
Regulatory
Environment
New Product Approvals
7 of 60 Total FDA Drug Approvals in 2021
Were Ophthalmic Drugs¹ and 1 in 2022
Therapeutix™
Dextenza
*SNDA
(dexamethasone ophthalmic insert) 0.4mg
for intracanalicular use
Roche
SuSvimo
ranibizumab injection For Ocular Implant
100
Roche
VABYSMO
faricimab-svoa injection 6 mg
Source:1.Endpoint Dec 29, 2021- Hitting a new record on drug approvals, the FDA offers a thumbs-up to another atopic dermatitis contender; OIS Year in Review 2021; Company press releases
OYSTER POINT
PHARMA
Odp
Santen
Verkazia 1 mg/mL
Allergan.
an AbbVie company
Vuity.
BAUSCH- Health
XIPERE
(triamcinolone acetonide
injectable suspension) 40 mg/mL
Ovut
SAMSUNG
BIOEPIS
BYOOVIZ™
Ranibizumab biosimilar
Ocuphire
PHARMAView entire presentation