Immix Biopharma Investor Presentation Deck
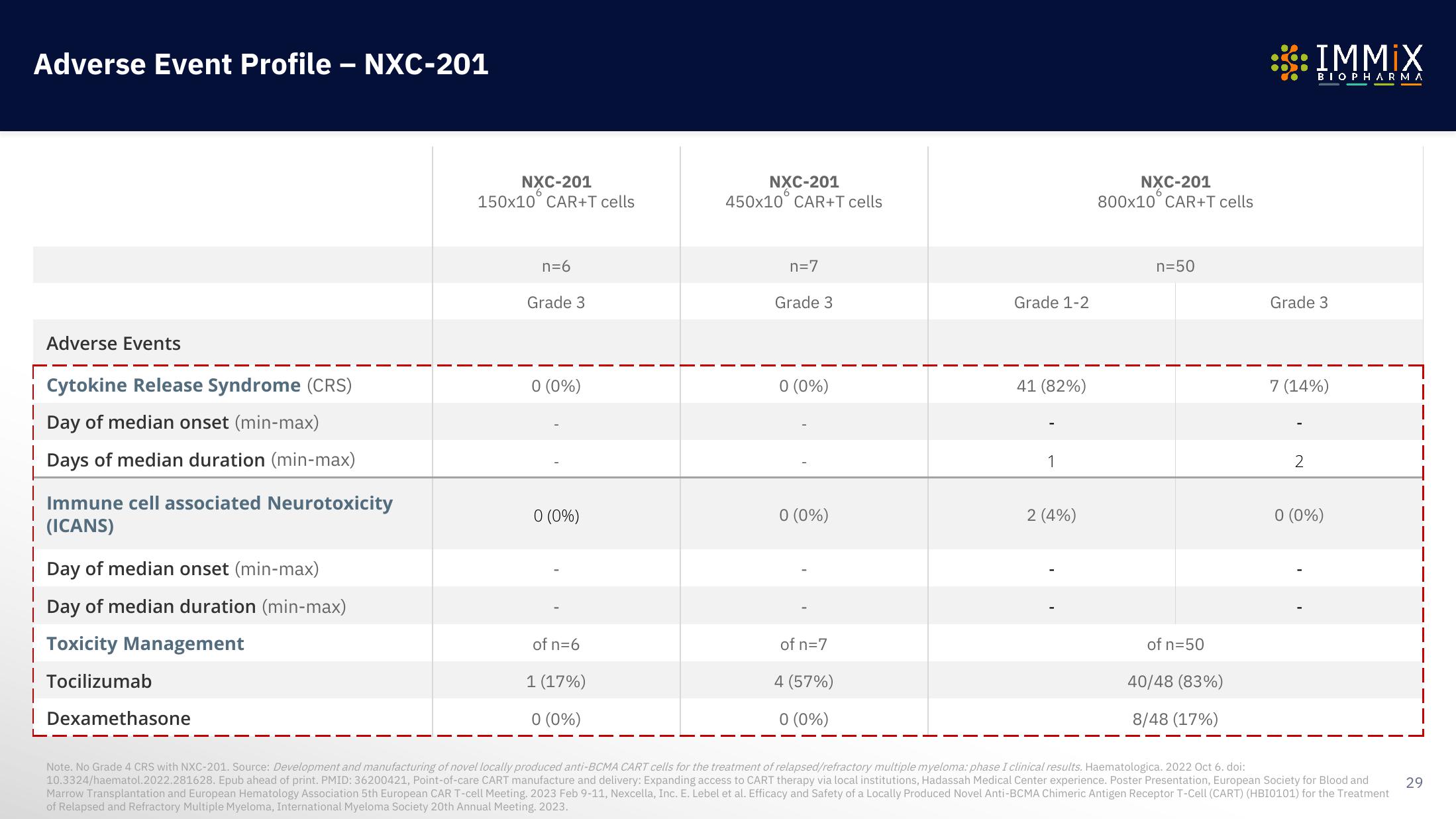
Adverse Event Profile - NXC-201
Adverse Events
Cytokine Release Syndrome (CRS)
Day of median onset (min-max)
Days of median duration (min-max)
Immune cell associated Neurotoxicity
(ICANS)
Day of median onset (min-max)
Day of median duration (min-max)
Toxicity Management
| Tocilizumab
| Dexamethasone
NXC-201
150x10 CAR+T cells
n=6
Grade 3
0 (0%)
0 (0%)
of n=6
1 (17%)
0 (0%)
NXC-201
450x10 CAR+T cells
n=7
Grade 3
0 (0%)
0 (0%)
of n=7
4 (57%)
0 (0%)
Grade 1-2
41 (82%)
1
2 (4%)
NXC-201
800x10 CAR+T cells
n=50
of n=50
40/48 (83%)
8/48 (17%)
●●●
IMMIX
S BIOPHARMA
Grade 3
7 (14%)
2
0 (0%)
Note. No Grade 4 CRS with NXC-201. Source: Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi:
10.3324/haematol.2022.281628. Epub ahead of print. PMID: 36200421, Point-of-care CART manufacture and delivery: Expanding access to CART therapy via local institutions, Hadassah Medical Center experience. Poster Presentation, European Society for Blood and
Marrow Transplantation and European Hematology Association 5th European CAR T-cell Meeting. 2023 Feb 9-11, Nexcella, Inc. E. Lebel et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment
of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting. 2023.
29View entire presentation