Ocuphire Pharma Investor Day Presentation Deck
DR
DME
ETDRS Severity
Level
Steps
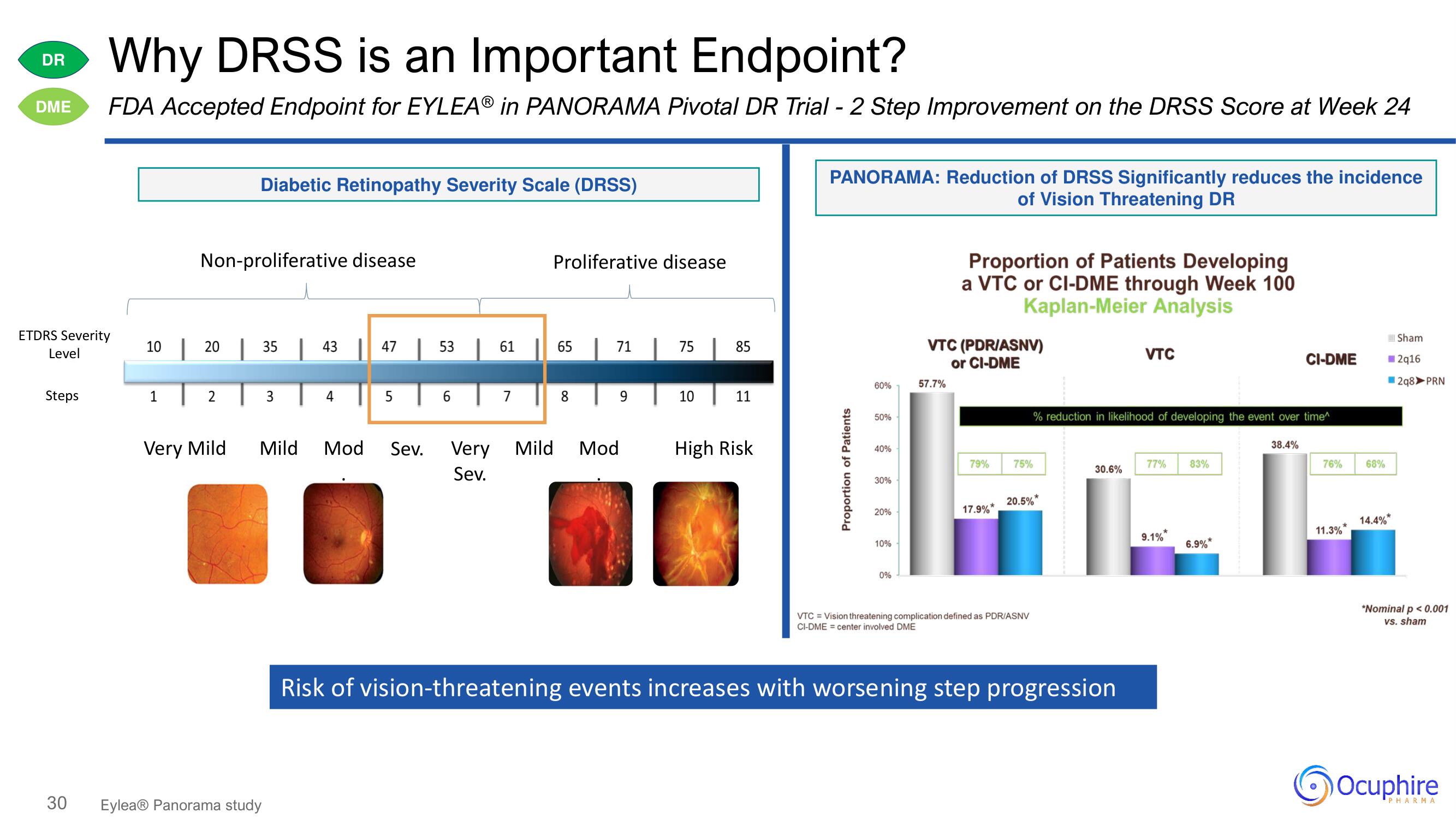
Why DRSS is an Important Endpoint?
FDA Accepted Endpoint for EYLEA® in PANORAMA Pivotal DR Trial - 2 Step Improvement on the DRSS Score at Week 24
30
10 | 20
1
Non-proliferative disease
2
Diabetic Retinopathy Severity Scale (DRSS)
Very Mild
35 43
3
Mild
Eylea® Panorama study
4
Mod
47
5
53
6
61
Proliferative disease
65
8
71
Sev. Very Mild Mod
Sev.
9
75
10
85
11
High Risk
PANORAMA: Reduction of DRSS Significantly reduces the incidence
of Vision Threatening DR
Proportion of Patients
60%
50%
40%
30%
20%
10%
0%
Proportion of Patients Developing
a VTC or CI-DME through Week 100
Kaplan-Meier Analysis
VTC (PDR/ASNV)
or CI-DME
57.7%
79% 75%
17.9%
20.5%
VTC = Vision threatening complication defined as PDR/ASNV
CI-DME = center involved DME
% reduction in likelihood of developing the event over time^
30.6%
VTC
Risk of vision-threatening events increases with worsening step progression
77% 83%
9.1%
6.9%
CI-DME
38.4%
76%
11.3%
68%
14.4%*
Sham
2q16
2q8 PRN
*Nominal p < 0.001
vs. sham
Ocuphire
PHARMAView entire presentation