Compass Therapeutics Investor Presentation Deck
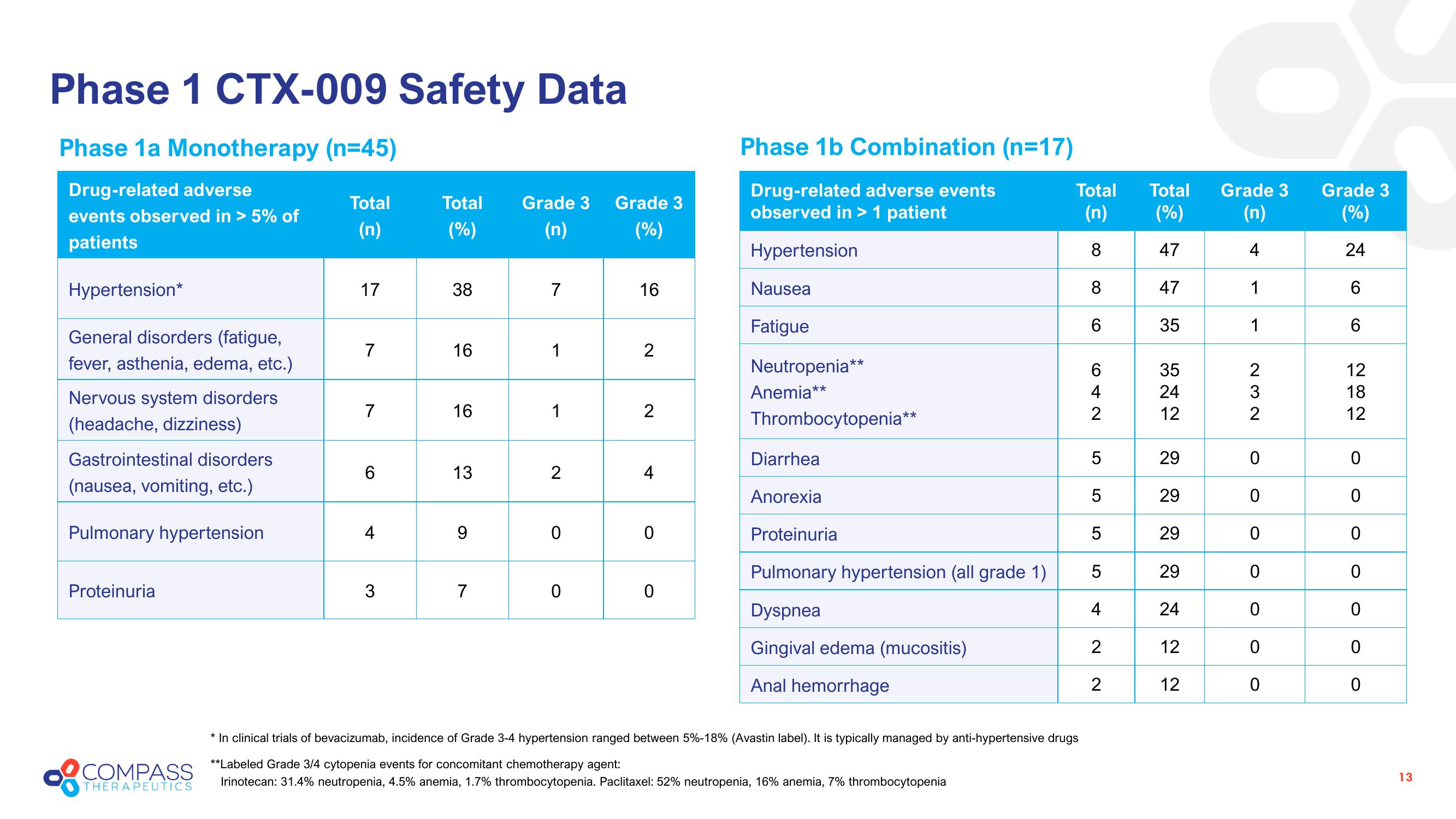
Phase 1 CTX-009 Safety Data
Phase 1a Monotherapy (n=45)
Drug-related adverse
events observed in > 5% of
patients
Hypertension*
General disorders (fatigue,
fever, asthenia, edema, etc.)
Nervous system disorders
(headache, dizziness)
Gastrointestinal disorders
(nausea, vomiting, etc.)
Pulmonary hypertension
Proteinuria
Total
(n)
17
7
7
6
4
3
Total Grade 3
(%)
(n)
38
16
16
13
9
7
7
1
1
2
0
0
Grade 3
(%)
16
2
2
4
0
0
Phase 1b Combination (n=17)
Drug-related adverse events
observed in > 1 patient
Hypertension
Nausea
Fatigue
Neutropenia**
Anemia**
Thrombocytopenia**
Diarrhea
Anorexia
Proteinuria
Pulmonary hypertension (all grade 1)
Dyspnea
Gingival edema (mucositis)
Anal hemorrhage
Total
(n)
8
8
6
* In clinical trials of bevacizumab, incidence of Grade 3-4 hypertension ranged between 5%-18% (Avastin label). It is typically managed by anti-hypertensive drugs
**Labeled Grade 3/4 cytopenia events for concomitant chemotherapy agent:
COMPASS
THERAPEUTICS Irinotecan: 31.4% neutropenia, 4.5% anemia, 1.7% thrombocytopenia. Paclitaxel: 52% neutropenia, 16% anemia, 7% thrombocytopenia
642
5
5
5
5
4
2
2
Total
(%)
47
47
35
5425
35
12
29
29
29
29
24
12
12
Grade 3
(n)
4
1
1
2
3
2
0
0
0
0
0
0
0
Grade 3
(%)
24
6
6
282
12
18
12
0
0
0
0
0
0
0
13View entire presentation