argenx SE Investor Day Presentation Deck
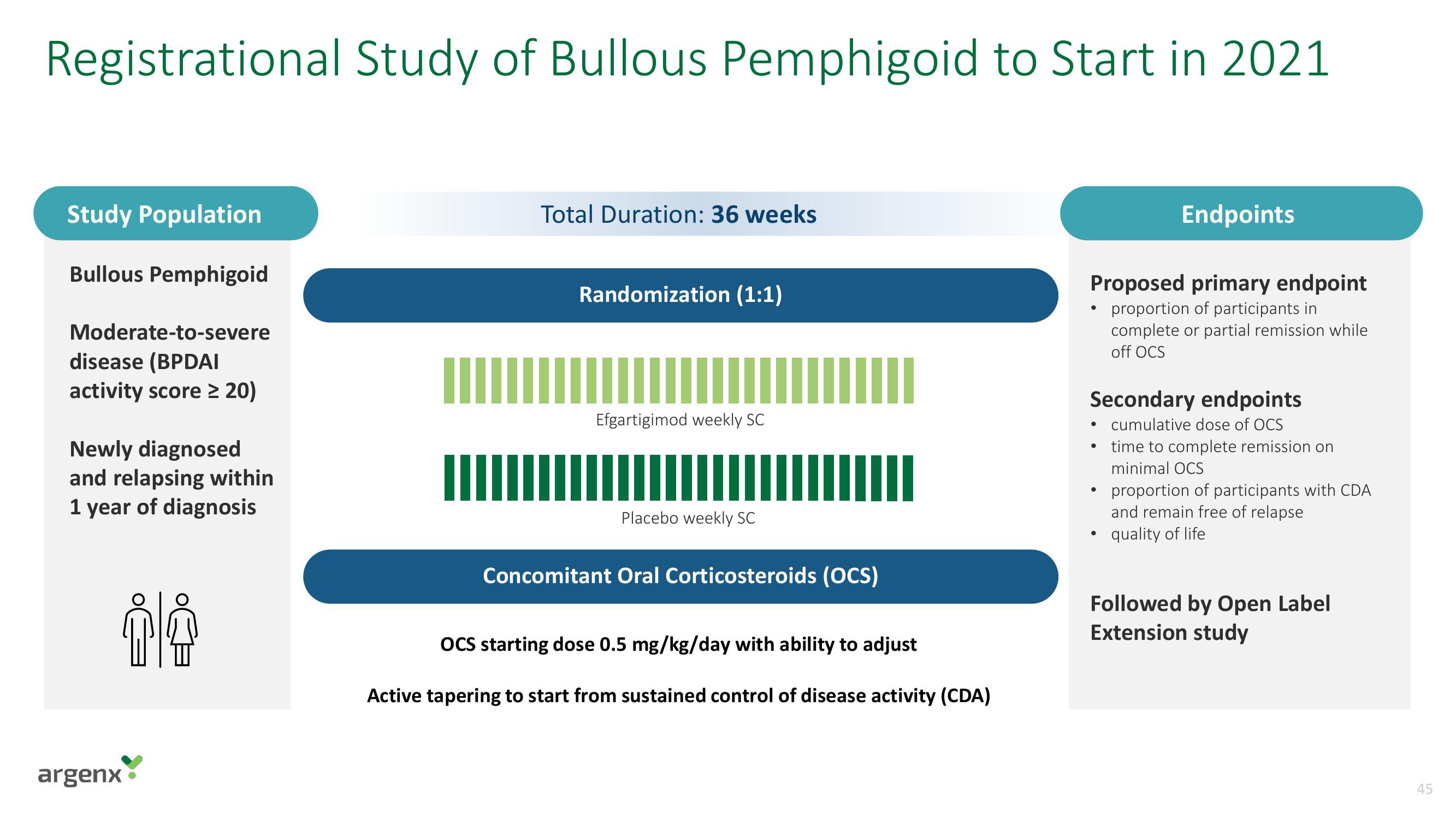
Registrational Study of Bullous Pemphigoid to Start in 2021
Study Population
Bullous Pemphigoid
Moderate-to-severe
disease (BPDAI
activity score ≥ 20)
Newly diagnosed
and relapsing within
1 year of diagnosis
ili
argenx
IIII
III
Total Duration: 36 weeks
Randomization (1:1)
III
Efgartigimod weekly SC
Placebo weekly SC
|||||
Concomitant Oral Corticosteroids (OCS)
OCS starting dose 0.5 mg/kg/day with ability to adjust
Active tapering to start from sustained control of disease activity (CDA)
Proposed primary endpoint
proportion of participants in
complete or partial remission while
off OCS
●
Endpoints
Secondary endpoints
• cumulative dose of OCS
• time to complete remission on
minimal OCS
●
proportion of participants with CDA
and remain free of relapse
quality of life
Followed by Open Label
Extension study
45View entire presentation