Ocuphire Pharma Investor Updates
P
18
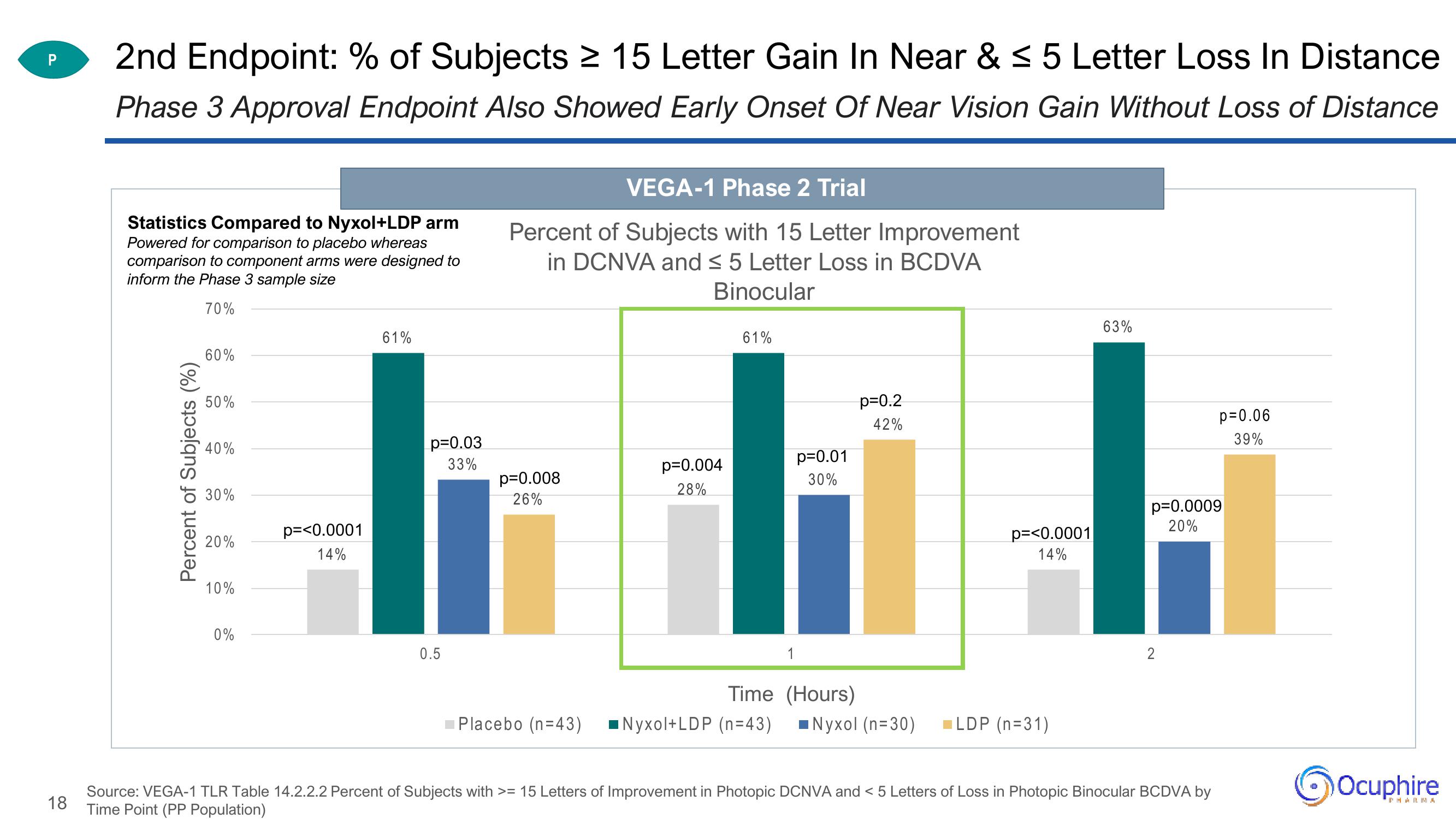
2nd Endpoint: % of Subjects ≥ 15 Letter Gain In Near & ≤ 5 Letter Loss In Distance
Phase 3 Approval Endpoint Also Showed Early Onset Of Near Vision Gain Without Loss of Distance
Statistics Compared to Nyxol+LDP arm
Powered for comparison to placebo whereas
comparison to component arms were designed to
inform the Phase 3 sample size
70%
Percent of Subjects (%)
60%
50%
40%
30%
20%
10%
0%
p=<0.0001
14%
61%
p=0.03
33%
0.5
VEGA-1 Phase 2 Trial
Percent of Subjects with 15 Letter Improvement
in DCNVA and ≤ 5 Letter Loss in BCDVA
Binocular
p=0.008
26%
Placebo (n=43)
p=0.004
28%
61%
Time
Nyxol+LDP (n=43)
1
p=0.01
30%
p=0.2
42%
(Hours)
Nyxol (n=30)
p=<0.0001
14%
LDP (n=31)
63%
p=0.0009
20%
2
p=0.06
39%
Source: VEGA-1 TLR Table 14.2.2.2 Percent of Subjects with >= 15 Letters of Improvement in Photopic DCNVA and < 5 Letters of Loss in Photopic Binocular BCDVA by
Time Point (PP Population)
Ocuphire
PHARMAView entire presentation