BenevolentAI Investor Conference Presentation Deck
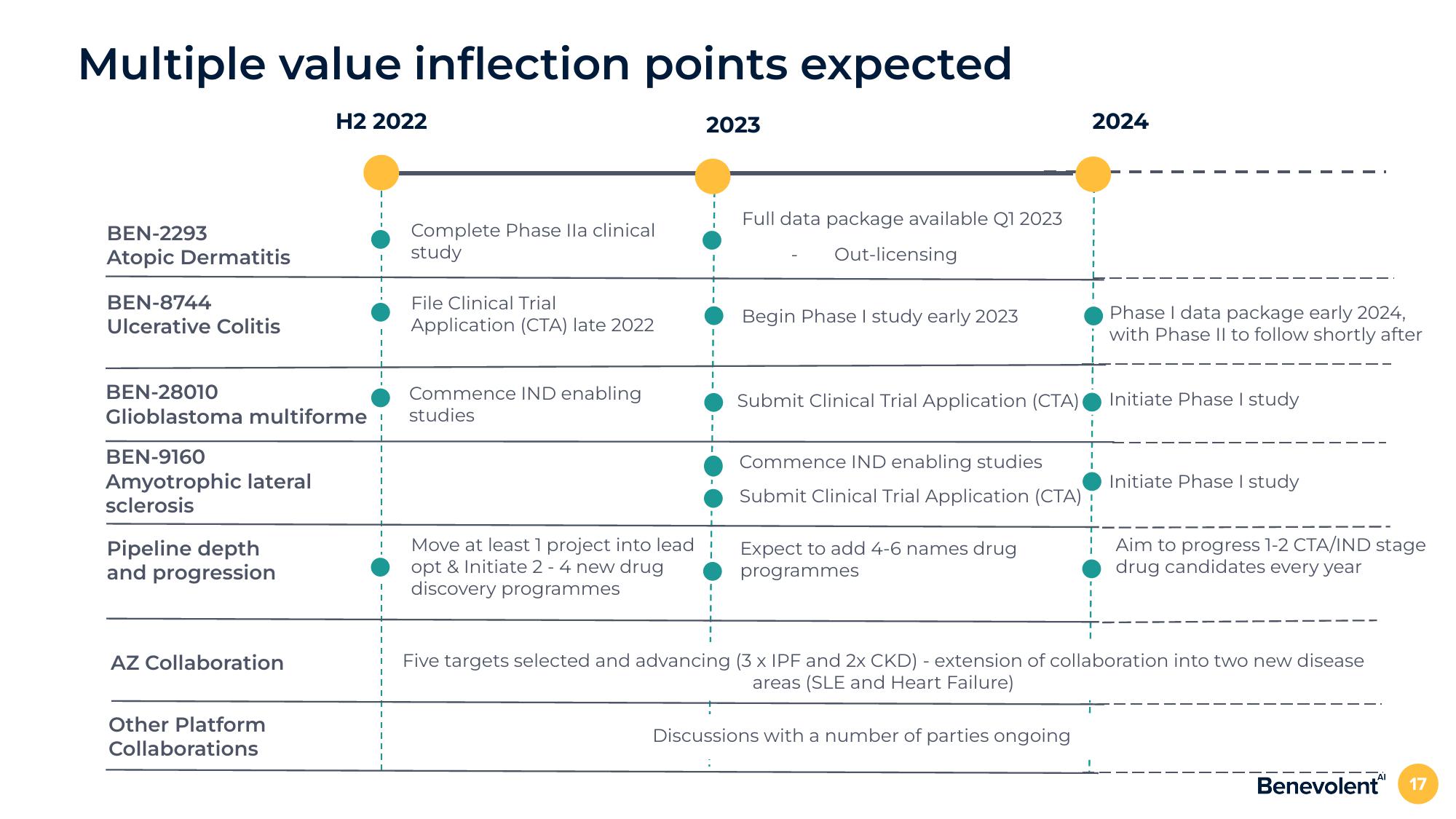
Multiple value inflection points expected
H2 2022
BEN-2293
Atopic Dermatitis
BEN-8744
Ulcerative Colitis
BEN-28010
Glioblastoma multiforme
BEN-9160
Amyotrophic lateral
sclerosis
Pipeline depth
and progression
AZ Collaboration
Other Platform
Collaborations
I
Complete Phase lla clinical
study
File Clinical Trial
Application (CTA) late 2022
Commence IND enabling
studies
Move at least 1 project into lead
opt & Initiate 2 - 4 new drug
discovery programmes
2023
I
Full data package available Q1 2023
Out-licensing
Begin Phase I study early 2023
Submit Clinical Trial Application (CTA)
Commence IND enabling studies
Submit Clinical Trial Application (CTA)
Expect to add 4-6 names drug
programmes
2024
Discussions with a number of parties ongoing
1
Phase I data package early 2024,
with Phase II to follow shortly after
Initiate Phase I study
Initiate Phase I study
Aim to progress 1-2 CTA/IND stage
drug candidates every year
Five targets selected and advancing (3 x IPF and 2x CKD) - extension of collaboration into two new disease
areas (SLE and Heart Failure)
Benevolent
17View entire presentation