BioNTech Investor Day Presentation Deck
Ⓒ
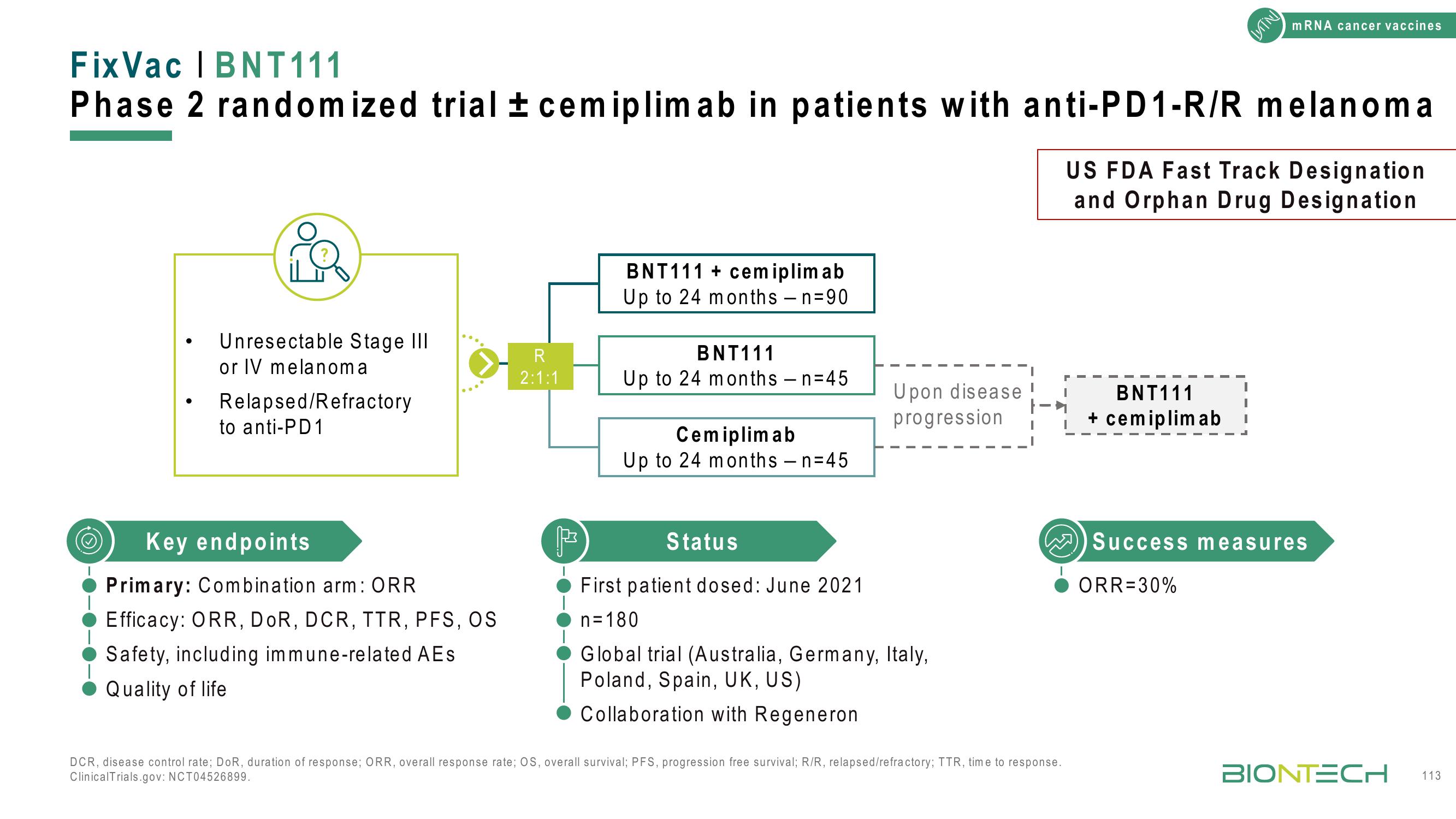
Fix Vac | BNT111
Phase 2 randomized trial ± cemiplimab in patients with anti-PD1-R/R melanoma
Unresectable Stage III
or IV melanoma
Relapsed/Refractory
to anti-PD1
Key endpoints
Primary: Combination arm: ORR
Efficacy: ORR, DOR, DCR, TTR, PFS, OS
Safety, including immune-related AEs
Quality of life
R
2:1:1
T
BNT111 + cemiplimab
Up to 24 months - n=90
BNT111
Up to 24 months - n=45
Cemiplimab
Up to 24 months - n=45
Upon disease
progression
Status
First patient dosed: June 2021
n=180
Global trial (Australia, Germany, Italy,
Poland, Spain, UK, US)
Collaboration with Regeneron
DCR, disease control rate; DoR, duration of response; ORR, overall response rate; OS, overall survival; PFS, progression free survival; R/R, relapsed/refractory; TTR, time to response.
Clinical Trials.gov: NCT04526899.
NUM
mRNA cancer vaccines
BNT111
+ cemiplimab
US FDA Fast Track Designation
and Orphan Drug Designation
Success measures
ORR=30%
BIONTECH 113View entire presentation