Evotec Investor Day Presentation Deck
evotec
Phase II data support best-in-class potential
P2X3 antagonist - Eliapixant (BAY1817080) Refractory Chronic Cough (RCC)
Safety
• Low rates of AEs including taste-
related AEs
PAGE 35
. All taste-related AEs mild and
resolved after cessation of therapy
Efficacy
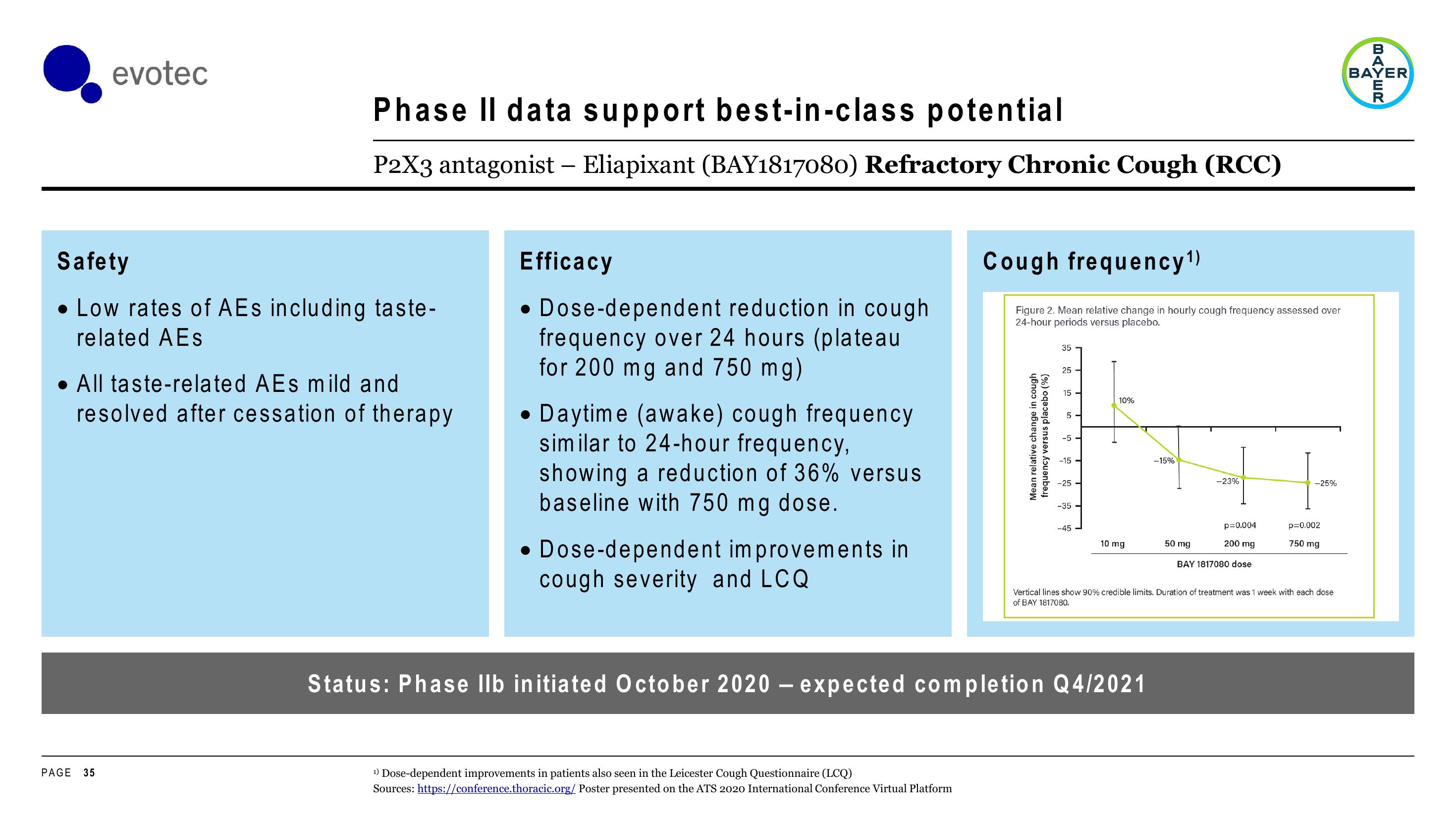
• Dose-dependent reduction in cough
frequency over 24 hours (plateau
for 200 mg and 750 mg)
Daytime (awake) cough frequency
similar to 24-hour frequency,
showing a reduction of 36% versus
baseline with 750 mg dose.
• Dose-dependent improvements in
cough severity and LCQ
Cough frequency¹)
Figure 2. Mean relative change in hourly cough frequency assessed over
24-hour periods versus placebo.
¹) Dose-dependent improvements in patients also seen in the Leicester Cough Questionnaire (LCQ)
Sources: https://conference.thoracic.org/ Poster presented on the ATS 2020 International Conference Virtual Platform
Mean relative change in cough
frequency versus placebo (%)
35
25
15
4.0 4.0
5
-5
-15
-25
-35
-45
10%
10 mg
Status: Phase Ilb initiated October 2020 - expected completion Q4/2021
-15%
-23%
p=0.004
200 mg
BAY 1817080 dose
50 mg
-25%
p=0.002
750 mg
Vertical lines show 90% credible limits. Duration of treatment was 1 week with each dose
of BAY 1817080.
BAYER
BAYERView entire presentation