Bionomics IPO Presentation Deck
·
wwwww
BNC210's Rapid Onset of Action is Potentially Well-Positioned for Social Anxiety Disorder
Emerging Regulatory Landscape & Unmet Need
No fast-acting FDA-approved medications for
as-needed treatment of SAD
Benzodiazepines prescribed off-label have
significant side effects of sedation, cognitive
impairment and potential for addiction
Growing unmet need based on improving
awareness and evolving social dynamics
FDA precedent on simplified public speaking
challenge endpoint for acute anxiety reduction
vs. placebo*
Bionomics
"Based on path of CNS peer proceeding with registrational Phose 3 endpoint
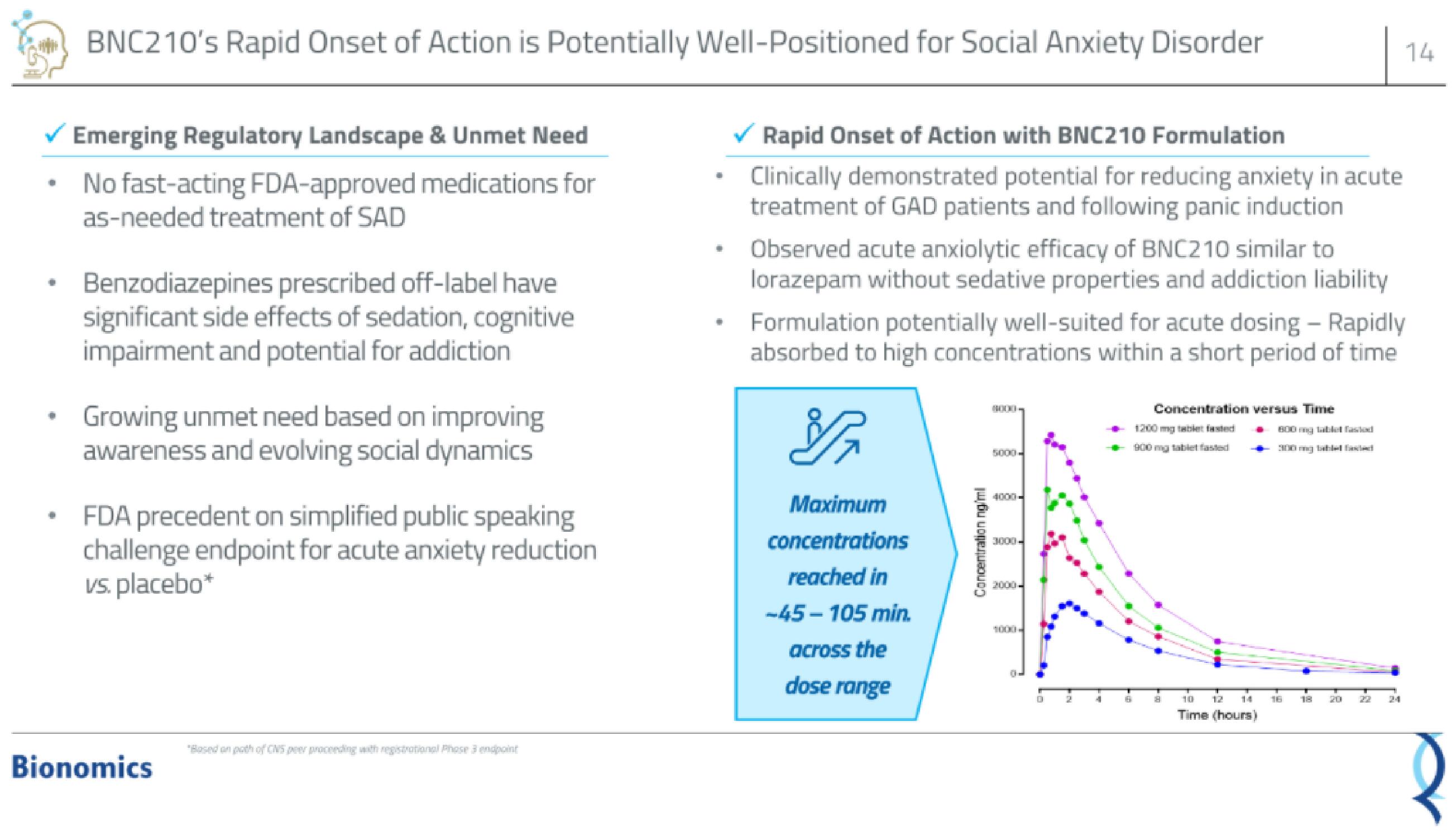
Rapid Onset of Action with BNC210 Formulation
Clinically demonstrated potential for reducing anxiety in acute
treatment of GAD patients and following panic induction
Observed acute anxiolytic efficacy of BNC210 similar to
lorazepam without sedative properties and addiction liability
Formulation potentially well-suited for acute dosing - Rapidly
absorbed to high concentrations within a short period of time
√
Maximum
concentrations
reached in
-45-105 min.
across the
dose range
Concentration ng/ml
5000-
4000-
D
T
2
E
161
Concentration versus Time
1200 mg tablert fasted
900 mg tablet fasted
1:8
T
10
Time (hours)
800 mg tablet fo
16
T
18
T
20
14
TView entire presentation