AstraZeneca Results Presentation Deck
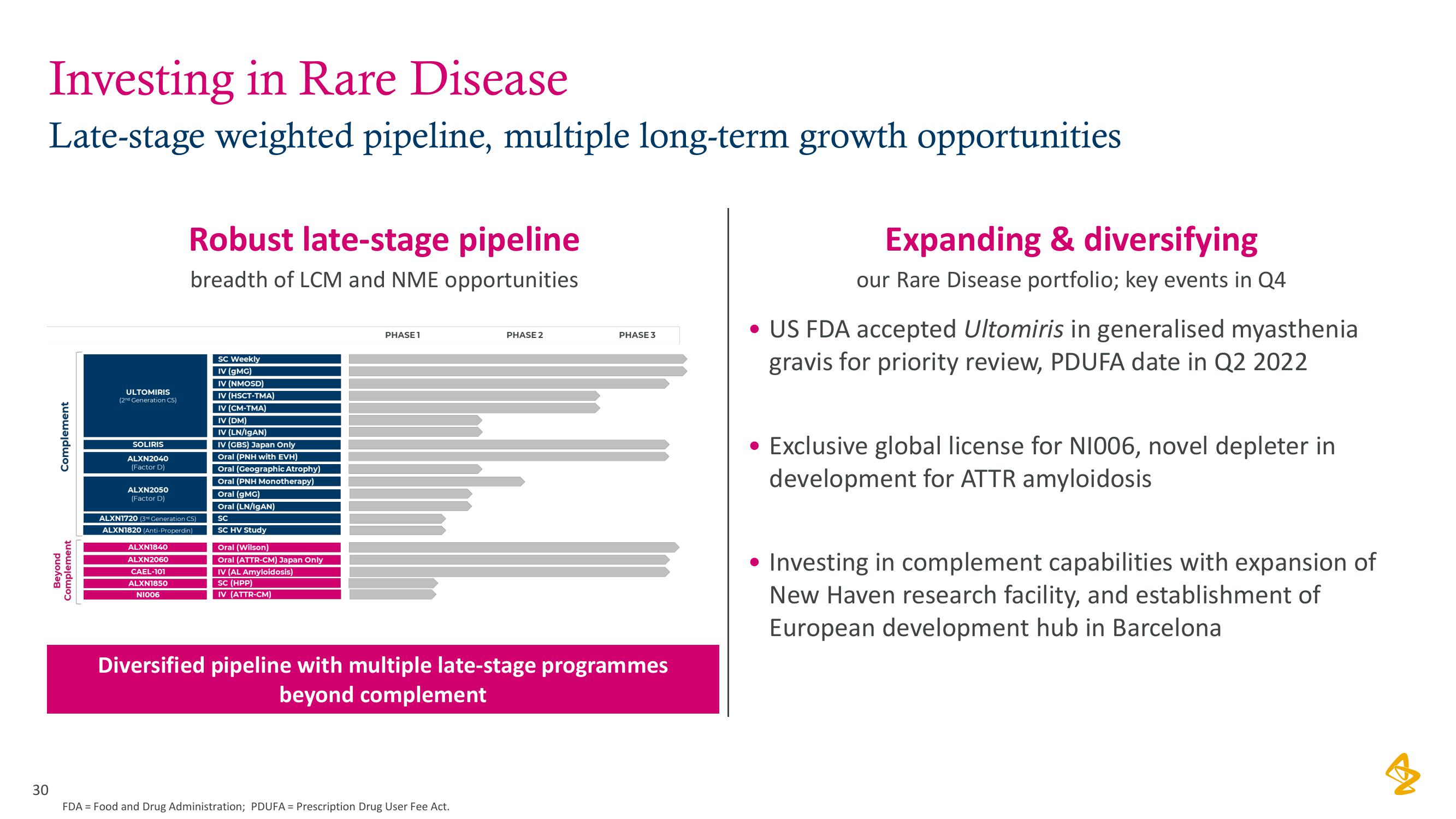
Investing in Rare Disease
Late-stage weighted pipeline, multiple long-term growth opportunities
30
Complement
Beyond
Complement
ULTOMIRIS
(2nd Generation C5)
SOLIRIS
ALXN2040
(Factor D)
ALXN2050
(Factor D)
Robust late-stage pipeline
breadth of LCM and NME opportunities
ALXN1720 (3rd Generation CS)
ALXN1820 (Anti-Properdin)
ALXN1840
ALXN2060
CAEL-101
ALXN1850
N1006
SC Weekly
IV (gMG)
IV (NMOSD)
IV (HSCT-TMA)
IV (CM-TMA)
IV (DM)
IV (LN/IGAN)
IV (GBS) Japan Only
Oral (PNH with EVH)
Oral (Geographic Atrophy)
Oral (PNH Monotherapy)
Oral (gMG)
Oral (LN/IgAN)
SC
SC HV Study
Oral (Wilson)
Oral (ATTR-CM) Japan Only
IV (AL Amyloidosis)
SC (HPP)
IV (ATTR-CM)
PHASE 1
PHASE 2
FDA = Food and Drug Administration; PDUFA = Prescription Drug User Fee Act.
PHASE 3
Diversified pipeline with multiple late-stage programmes
beyond complement
Expanding & diversifying
our Rare Disease portfolio; key events in Q4
• US FDA accepted Ultomiris in generalised myasthenia
gravis for priority review, PDUFA date in Q2 2022
Exclusive global license for N1006, novel depleter in
development for ATTR amyloidosis
Investing in complement capabilities with expansion of
New Haven research facility, and establishment of
European development hub in Barcelona.
BView entire presentation