Valneva IPO Presentation Deck
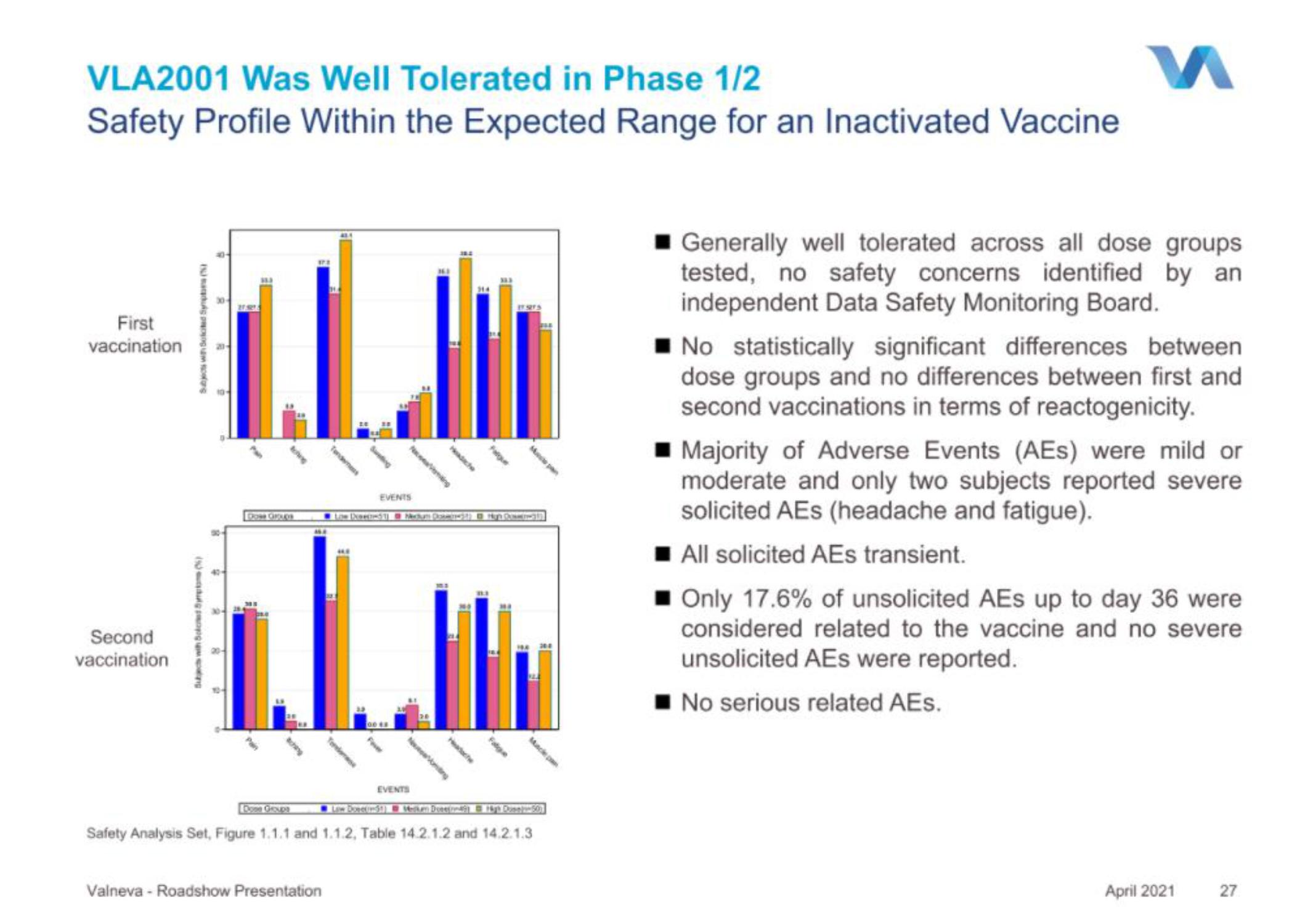
VLA2001 Was Well Tolerated in Phase 1/2
Safety Profile Within the Expected Range for an Inactivated Vaccine
First
vaccination
Second
vaccination
(5) aug
Subjects with Sold Byro (5)
201-
10-
DOW GROULOS
aching
Lab
Tenderness
Valneva - Roadshow Presentation
Terem
EVENTS
Low Deere Neu
Falque
JM
Fatge
EVENTS
| Low Doseondt) ■ Vestum Dosef49) © High Dưeep-501
Safety Analysis Set, Figure 1.1.1 and 1.1.2, Table 14.2.1.2 and 14.2.1.3
M
Generally well tolerated across all dose groups
tested, no safety concerns identified by an
independent Data Safety Monitoring Board.
No statistically significant differences between
dose groups and no differences between first and
second vaccinations in terms of reactogenicity.
Majority of Adverse Events (AES) were mild or
moderate and only two subjects reported severe
solicited AEs (headache and fatigue).
■All solicited AEs transient.
■ Only 17.6% of unsolicited AEs up to day 36 were
considered related to the vaccine and no severe
unsolicited AEs were reported.
No serious related AEs.
April 2021
27View entire presentation