BioAtla Investor Presentation Deck
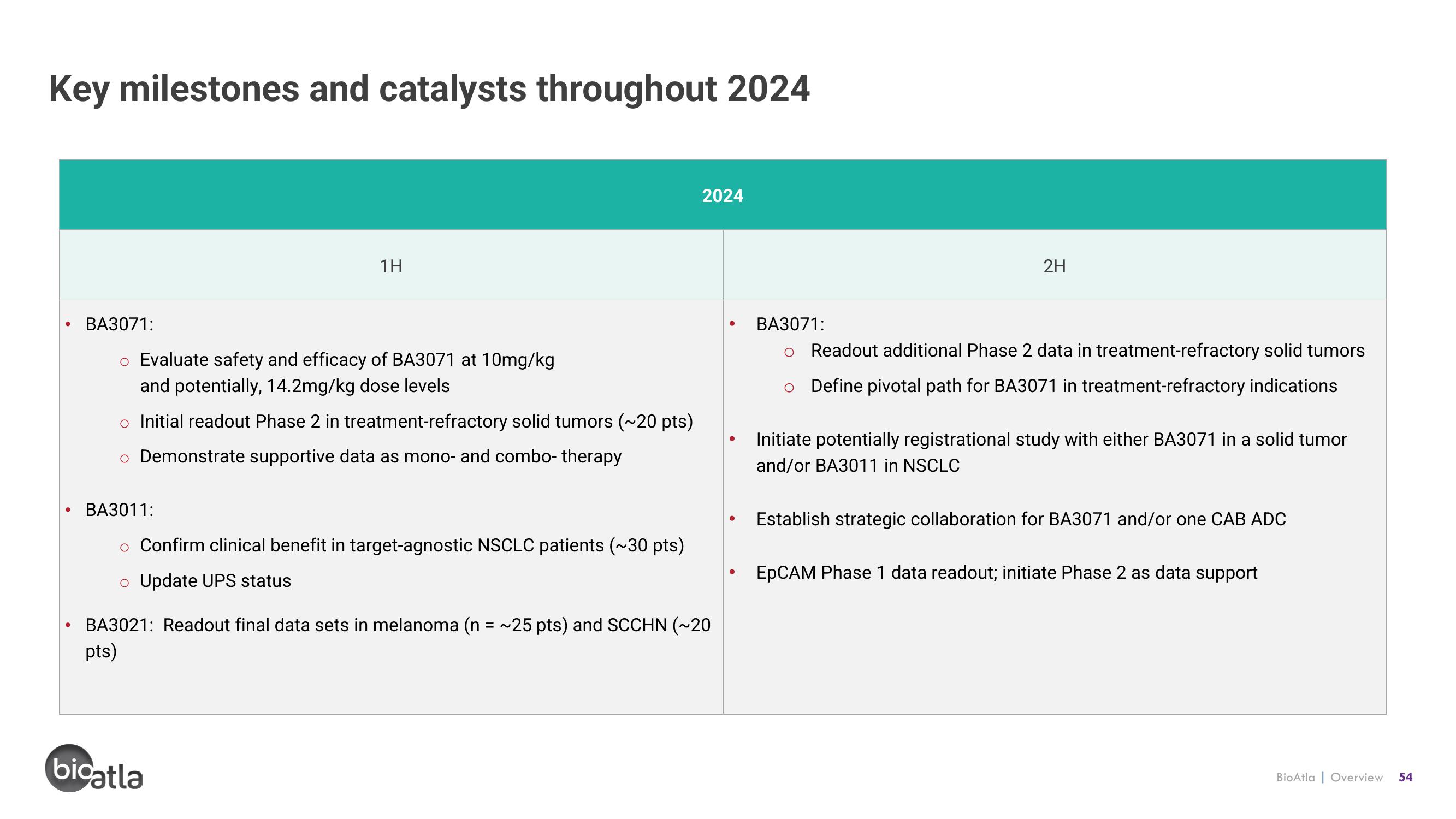
Key milestones and catalysts throughout 2024
1H
BA3071:
o Evaluate safety and efficacy of BA3071 at 10mg/kg
and potentially, 14.2mg/kg dose levels
o Initial readout Phase 2 in treatment-refractory solid tumors (~20 pts)
o Demonstrate supportive data as mono- and combo- therapy
BA3011:
o Confirm clinical benefit in target-agnostic NSCLC patients (~30 pts)
o Update UPS status
bicatla
2024
BA3021: Readout final data sets in melanoma (n = ~25 pts) and SCCHN (~20
pts)
BA3071:
2H
Readout additional Phase 2 data in treatment-refractory solid tumors
O Define pivotal path for BA3071 in treatment-refractory indications
Initiate potentially registrational study with either BA3071 in a solid tumor
and/or BA3011 in NSCLC
Establish strategic collaboration for BA3071 and/or one CAB ADC
EpCAM Phase 1 data readout; initiate Phase 2 as data support
BioAtla| Overview 54View entire presentation