Valneva IPO Presentation Deck
VLA2001 Was Highly Immunogenic in Phase 1/2
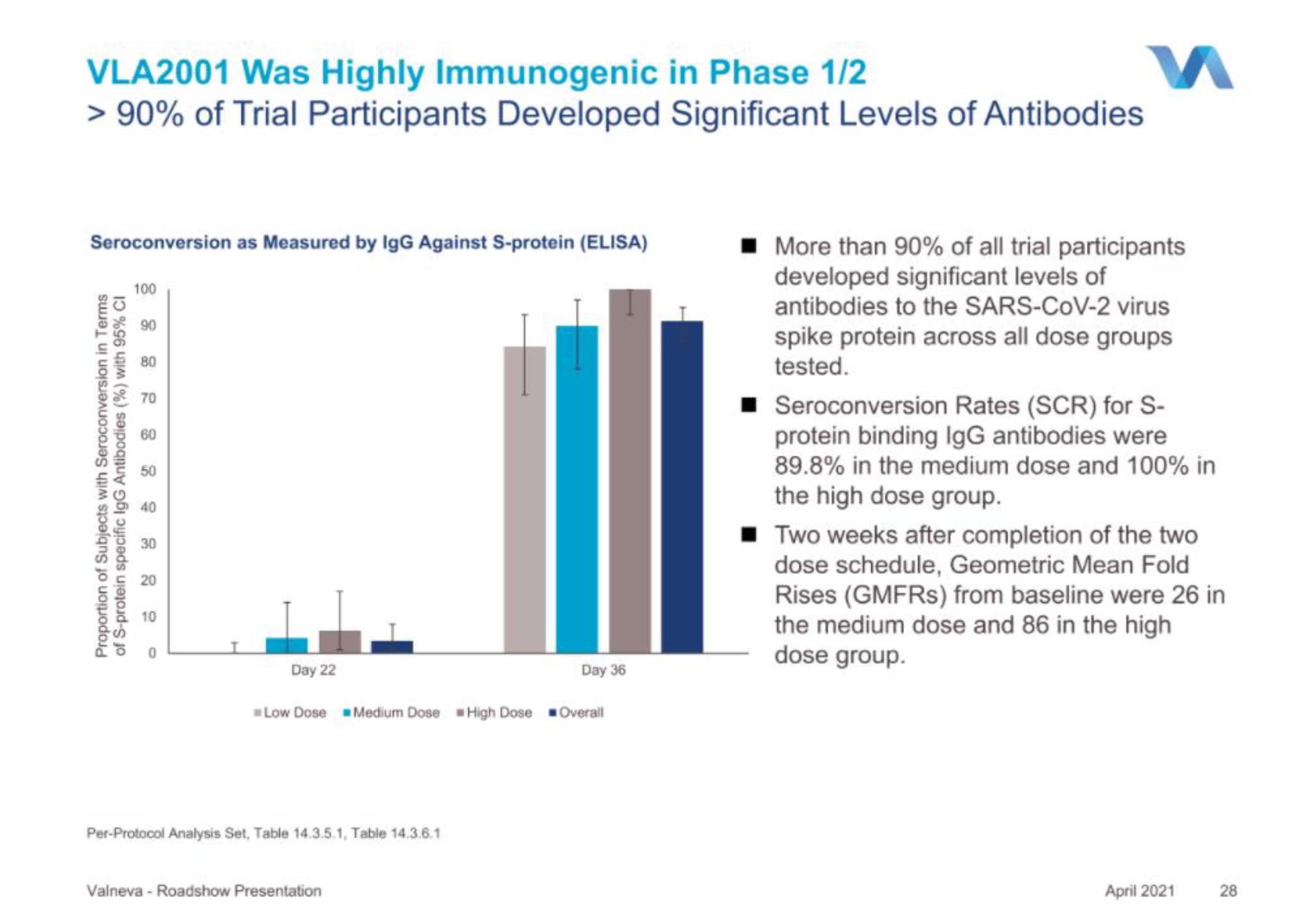
> 90% of Trial Participants Developed Significant Levels of Antibodies
Seroconversion as Measured by IgG Against S-protein (ELISA)
Proportion of Subjects with Seroconversion in Terms
of S-protein specific IgG Antibodies (%) with 95% CI
100
90
80
70
60
40
30
20
10
0
Day 22
Low Dose Medium Dose High Dose Overall
Per-Protocol Analysis Set, Table 14.3.5.1, Table 14.3.6.1
Valneva - Roadshow Presentation
Day 36
■ More than 90% of all trial participants
developed significant levels of
antibodies to the SARS-CoV-2 virus
spike protein across all dose groups
tested.
■ Seroconversion Rates (SCR) for S-
protein binding IgG antibodies were
89.8% in the medium dose and 100% in
the high dose group.
Two weeks after completion of the two
dose schedule, Geometric Mean Fold
Rises (GMFRS) from baseline were 26 in
the medium dose and 86 in the high
dose group.
April 2021
28View entire presentation